Photo credits: ScenTree SAS
Bourgeonal™
Floral > Light Flowers > Aldehydes > Aquatic > Green
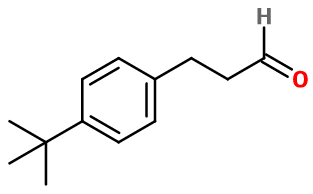
Burgenal ; 3-(4-tert-butylphenyl)propanal ; 4-(1,1-dimethylethyl)-benzenepropanal ; Para-tertbutyl dihydrocinnamaldehyde ; Para-tertbutyl dihydrocinnamic aldehyde ; 3-(4-tert-butylphenyl)propionaldehyde ; 4-(1,1-dimethyl ethyl) benzene propanal ; Langeonal ; Lilional ; Liliphenal ; 3-[4-(2-methyl-2-propanyl)phenyl]propanal

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Bourgeonal - 30gr | - |
Visit website
|
- | - | - | |
|
|
LIGEONAL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
18127-01-0 -
EINECS number :
242-016-2 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Heart -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,961 -
Refractive Index @20°C :
1.508 - 1.512 @20°C -
Optical rotation :
Data not available. -
Vapor pressure :
0.0015 mmHg @20°C 0.009 mmHg @25°C -
Flash Point :
73°C (163,4°F)
-
Molecular formula :
C13H18O -
Molecular Weight :
190,28 g/mol -
Log P :
3 -
Fusion Point :
-11°C (12,2°F) -
Boiling Point :
207°C (404,6°F) -
Detection Threshold :
0,4 ng/l air
Chemistry & Uses
Uses in perfumery :
Bourgeonal™ is used in all types of perfumery, in floral-aldehydic, green notes. Often used for its stability in soap and detergent bases, in addition to alcoholic perfumery.
Year of discovery :
1959
Natural availability :
Bourgeonal™ is not available in its natural state.
Isomerism :
The meta and ortho isomers of Bourgeonal™ are not used in perfumery. Cyclamen Aldehyde is a positional isomer of Bourgeonal™. However, its smell is quite different, as it is more marine. Both have an aldehydic and floral-white flowers smell.
Synthesis precursor :
Bourgeonal™ forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Synthesis route :
Bourgeonal™ can be synthesized from 4-tert-Butyl benzaldehyde by an aldol reaction with Acetaldehyde. The intermediate product that is obtained is 4-tert-Butyl Cinnamaldehyde. A catalytic hydrogenation converts this intermediate into Bourgeonal™. Another synthetic route reacts 4-tert-Butyl benzene with acrolein diacetate in the presence of a Lewis acid. Then, the intermediate product is subjected to a saponification.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Exclusively stable in fabric conditioners, shampoos and hair conditioners.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,0041 % 0,025 % 0,025 % 0,47 % 0,12 % 0,029 % 0,037 % 0,0096 %0,087 % Cat.5A B C DCat.6 0,12 % 0,029 % 0,037 % 0,0096 %0,087 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,029 % 0,029 %0,0096 % 0,099 % 0,099 % 0,24 %0,0096 % 0,0096 %6,9 % Cat.10A BCat.11A BCat.12 0,099 % 0,24 %0,0096 % 0,0096 %6,9 %