Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Aldéhyde Cyclamen - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
CYCLAMEN ALDEHYDE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
103-95-7 -
EINECS number :
203-161-7 -
FEMA number :
2743 -
FLAVIS number :
05.045
-
JECFA number :
1465 -
Volatility :
Heart -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,948 -
Refractive Index @20°C :
1.503 - 1.508 -
Optical rotation :
Data not available. -
Vapor pressure :
0.00397 mmHg @20°C 0.009 mmHg @25°C -
Flash Point :
109°C (228,2°F)
-
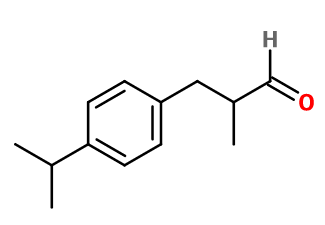
Molecular formula :
C13H18O -
Molecular Weight :
190,28 g/mol -
Log P :
3,4 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
270°C (518°F) -
Detection Threshold :
2,51 ng/l air
Chemistry & Uses
Uses in perfumery :
Cyclamen Aldehyde is used in marine and floral-green accords such as lily of the valley. Provides volume and an ozonic note.
Year of discovery :
Discovered in 1929. Patent N°1,844,013 published in October,18 1929 by Knorr.A and Weissenborn.A for Winthrop Chemical Company
Natural availability :
Cyclamen Aldehyde is not available in its natural state.
Isomerism :
Cyclamen Aldehyde has an asymmetric carbon. It is the racemic mixture of the molecule that is used in perfumery. The ortho and meta isomers of Aldehyde Cyclamen are not used. Damascenone-Beta is a constitutional isomer of Cyclamen Aldehyde. Both have a very different smell: Damascenone-Beta is reminiscent of cooked apples.
Synthesis precursor :
Cyclamen Aldehyde Schiff base by reaction with Methyl Anthranilate has a smell of lily of the valley and orange blossom. Several acetals may also be formed by reaction with alcohols in an acid medium and have an olfactory interest.
Synthesis route :
Cyclamen Aldehyde is synthesized by two different routes. The first is a condensation of two aldehydes: 4-isopropylbenzaldehyde and Acetaldehyde, with an excess of the first reagent and a slow addition of the second, to avoid self-condensation of the latter. This reaction is followed by a hydrogenation of the double bond formed during the process. The second synthetic route is a Friedel-Craft reaction between isopropylbenzene and methacrolein diacetate, followed by an acid hydrolysis of the intermediate product obtained.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Unstable in acidic products, except fabric conditioners, and in very alkaline products, such as bleach.
Other comments :
Cyclamen Aldehyde must not contain more than 1.5% cyclamen alcohol because it is an irritant.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,11 % 0,14 % 0,038 % 0,95 % 0,45 % 0,076 % 0,076 % 0,025 %0,076 % Cat.5A B C DCat.6 0,45 % 0,076 % 0,076 % 0,025 %0,076 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,076 % 0,076 %0,025 % 0,23 % 0,23 % 0,72 %0,025 % 0,025 %16 % Cat.10A BCat.11A BCat.12 0,23 % 0,72 %0,025 % 0,025 %16 %
-
Specified ingredients: notes
Cyclamen aldehyde should not contain more than 1.5% of Cyclamen alcohol.
Contributions from other sources
Cyclamen aldehyde has been found in natural extracts but only at trace levels.