Photo credits: ScenTree SAS
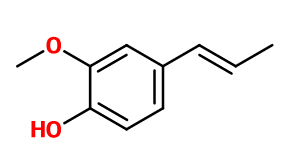
Isoeugenol
Spicy > Warm Spices > Eugenol > Yellow Fruits
2-methoxy-4-prop-1-en-2-ylphenol ; 4-hydroxy-3-methoxy-1-propen-1-yl benzene ; 4- hydroxy-3-methoxy-1-propenyl benzene ; 4- hydroxy-3-methoxypropenyl benzene ; 2-methoxy-4-(1-methylethenyl)phenol ; 2-methoxy-4-(1-methylvinyl)phenol ; 2-methoxy-4-(1-propenyl)phenol ; 3-methoxy-4-hydroxy-1-propen-1-yl benzene ; 2-methoxy-4-propenyl phenol ; 4-propenyl guaiacol

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Iso Eugénol - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
Isoeugenol Trans 92% | CL-702 |
Visit website
|
Natural |



|
800 Kgs | 92 |
|
|
Isoeugenol Trans 88% | CL-701 |
Visit website
|
Natural |



|
800 Kgs | 88 |
|
|
ISOEUGENOL | M_0053263 |
Visit website
|
Synthétique | - | - | |
|
|
ISOEUGENOL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
97-54-1 -
EINECS number :
202-590-7 -
FEMA number :
2468 -
FLAVIS number :
04.004
-
JECFA number :
1260 -
Volatility :
Heart -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Pinkish liquid -
Density :
1,087 -
Refractive Index @20°C :
1.572 - 1.577 @25°C -
Optical rotation :
Data not available. -
Vapor pressure :
0.003 mmHg @20°C -
Flash Point :
112°C (233,6°F)
-
Molecular formula :
C10H12O2 -
Molecular Weight :
164.20 g/mol -
Log P :
2,65 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
270°C (518°F) -
Detection Threshold :
100 ppb (0,00001%)
Chemistry & Uses
Uses in perfumery :
Isoeugenol is used in carnation, spicy and ambery notes, often combined with Eugenol. Often replaced by Acetyl-Isoeugenol, unregulated.
Year of discovery :
Data not available.
Natural availability :
In many cases, naturally present Isoeugenol is accompanied by Eugenol, which is present in greater quantities in the following raw materials. Isoeugenol can be extracted from Clove Bud EO (1%), betel leaf (about 10%) or Ylang-Ylang Extra EO, or other fractions (up to 0.5% depending on the fractions).
Isomerism :
Isoeugenol has two diastereomers with a similar smell. It is the mixture of these two isomers that is the most used in perfumery. The trans isomer is nevertheless the most present in this mixture, as it is thermodynamically more stable. Eugenol is an isomer of Isoeugenol, as the double bond present in the two molecules is simply relocated from one molecule to another. Its smell is less fruity but more woody and vanillic. Styrallyl acetate and Frambinone® are examples of constitutional isomers of Isoeugenol. Their smell is however very different.
Synthesis precursor :
Isoeugenol is a precursor to the synthesis of several compounds of olfactory interest. Catalytic hydrogenation of the molecule allows to obtain Dihydoeugenol. In the past, Vanillin was produced by an oxidation reaction on Isoeugenol. The alcohol function of the molecule can finally be used for several esterification reactions, to obtain Isoeugenyl acetate for example.
Synthesis route :
Isoeugenol can be produced from Eugenol. Forming sodium or potassium salts from Eugenol allows to isomerize the molecule by heating it in order to obtain Isoeugenol by an acid treatment. This isomerization can also be obtained directly by a ruthenium or rhodium catalysis.
Stability :
Becomes red under the effect of light. This raw material is not convenient in every functional base : can't be used in a candle or shower gel base.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,019 % 0,0057 % 0,12 % 0,11 % 0,027 % 0,027 % 0,027 % 0,009 %0,063 % Cat.5A B C DCat.6 0,027 % 0,027 % 0,027 % 0,009 %0,063 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,22 % 0,22 %0,009 % 0,21 % 0,21 % 0,75 %0,009 % 0,009 %No Restriction Cat.10A BCat.11A BCat.12 0,21 % 0,75 %0,009 % 0,009 %No Restriction


















