Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
MANDARIN ALDEHYDE 10 TEC | 965765 |
Visit website
|
Molecules | - | - | |
|
|
Aldéhyde Mandarine - 30gr | - |
Visit website
|
- | - | - | |
|
|
TRANS-2-DODECENAL | M_0062898 |
Visit website
|
Naturel | - | - | |
|
|
MANDARINE ALDEHYDE (10%CE) | - |
Visit website
|
- | 10 grs | - | |
|
|
TRANS 2 DODECENAL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
20407-84-5 -
EINECS number :
243-797-2 -
FEMA number :
2402 -
FLAVIS number :
05.144
-
JECFA number :
1350 -
Volatility :
Head/Heart -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Pale yellow liquid -
Density :
0,841 - 0,849 @25°C -
Refractive Index @20°C :
1.455 - 1.459 @20°C -
Optical rotation :
Data not available. -
Vapor pressure :
0.01 mmHg @20°C 0.0105 mmHg @25°C -
Flash Point :
113°C (235,4°F)
-
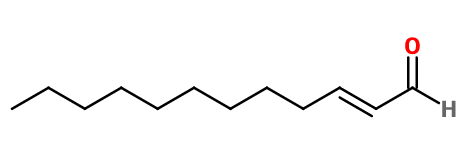
Molecular formula :
C12H22O -
Molecular Weight :
182,31 g/mol -
Log P :
4,53 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
126°C (258,8°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Mandarin Aldehyde is used for all types of perfumes, for fresh notes. Used in floral-aldehydic, coniferous, ambery notes, for a contribution of a characteristic aldehydic note. Brings freshness, boosts the head and gives tenacity to citrus notes.
Year of discovery :
Data not available.
Natural availability :
Mandarin Aldehyde can be extracted from Coriander Leaf EO in its natural state, where it is present at about 20%.
Isomerism :
Mandarin Aldehyde has two diastereoisomers (Z) and (E). The isomer (E) in most often used in perfumery, to the detriment of the isomer (Z). Concentration of trans isomer is minimum 93%
Synthesis precursor :
Mandarin Aldehyde forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Synthesis route :
Mandarin Aldehyde is an aliphatic aldehyde. As other aldehydes, it can be synthesized by reacting dodecenyl halides (chloride for example) with dimethyl sulfoxide (DMSO), followed by an alkaline treatment with sodium bicarbonate. Other synthesis routes exist using a Rosenmund reaction from dodecenic acid and an acid chloride.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Other comments :
comparing it to other aldehydesas Aldehyde C-12 lauric, Mandarin Aldehyde has a more fuity smell of Mandarin Yellow EO.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment