Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
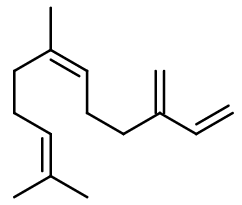
FARNESENE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
502-61-4 -
EINECS number :
207-948-6 -
FEMA number :
3839 -
FLAVIS number :
01.040
-
JECFA number :
1343 -
Volatility :
Head -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,861 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
110°C (230°F)
-
Molecular formula :
C15H24 -
Molecular Weight :
204,36 g/mol -
Log P :
7,1 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
-
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Farnesene is used to make fruity note greener, by bringing a quince note. It also brings a fruity and green note to floral accords.
Year of discovery :
Data not available.
Natural availability :
Many essential oils contain Farnesene isomers : Juniper Berry EO, Blue Chamomile EO, Lavender EO and Sambac Jasmine Absolute for example.
Isomerism :
Usually, Farnesene is used in perfumery as a mixture of isomers. Nevertheless, some plants contain precise isomers. Thus, Farnesene used in perfumery depends on the pant it is extracted from. For exemple, Ylang-Ylang III contains trans-alpha-Farnesene. Trans-beta-Farnesene is also to be distinguished, and can be found for example in Juniper Berry EO. Being a sesquiterpene, Farnesene is a constitutional isomer of Beta-Caryophyllene, Alpha-Cedrene and Valencene.
Synthesis precursor :
As any terpene, Farnesene can be used for the synthesis of many compounds, but it is not used for the synthesis of a compound used in perfumery.
Synthesis route :
Farnesene is rarely synthesized for its use in perfumery. It is usually extracted from plants as apple, perilla seed or ylang-ylang. A synthesis can nevertheless be considered, carrying out a Diels-Alder reaction, as for most terpenes.
Stability :
Terpenes tend to polymerize under the effect of high oxydation, and are unstable in alkaline and acidic bases.
Other comments :
Farnesene belongs to the terpenes family. Including 15 carbon atoms, it is a sesquiterpene, as Beta-Caryophyllene.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















