Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
CEDRENE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
469-61-4 -
EINECS number :
207-418-4 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
01.022
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,93 -
Refractive Index @20°C :
1.495 - 1.510 -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
104°C (219,2°F)
-
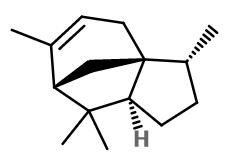
Molecular formula :
C15H24 -
Molecular Weight :
204,35 g/mol -
Log P :
Donnée indisponible. -
Fusion Point :
Donnée indisponible. -
Boiling Point :
262°C (503,6°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Alpha-Cedrene is generally used for leather notes or as a substitute for Cedarwood Virginia EO, for its substantially equivalent note.
Year of discovery :
Isolated by scientist Walter in 1841 (ex. Juniperus virginiana). In 1953, Stork and Breslow were the first to synthesize dl-cedrol and dl-Alpha-Cedrene. Two years later, they acheived in synthesizing completely cedrol and Alpha-Cedrene.
Natural availability :
Alpha-Cedrene is one of the major components of Cedarwood Virginia EO, Cedarwood Texas EO, Cedarwood Chinese EO, among others, and is present in trace amounts especially in Verbena EO.
Isomerism :
Alpha-Cedrene has several isomers, by delocalization of its double bond. We distinguish beta-Cedrene, which has a smell relatively similar to Alpha-Cedrene. Moreover, this molecule is a constitutional isomer of beta-Caryophyllene and Valencene, but has a smell much less spicy or green than these two isomers.
Synthesis precursor :
Alpha-Cedrene is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
There are several routes of synthesis for Alpha-Cedrene depending on the starting reagent. Only one of them is detailed below: One of the syntheses with the least steps is a Corey synthesis from 2-methylhept-2-enen lithiated in its carbon 6, reacting with 3-methoxycyclopent-2-enone. The obtained intermediate product undergoes a reduction with sodium borohydride, then a treatment with diiodomethane, catalysed by brass, and a pyrogenation in the presence of chromium trioxide and dichloromethane. An acetylation of the ketone function that is still present, allows to form a carbocation which, by rearrangement, forms epi-Alpha-Cedrene and Alpha-Cedrene.
Stability :
Terpenes tend to polymerize by oxydation
Other comments :
Alpha-Cedrene is a sesquiterpene. This means that it has 15 carbon atoms, linked to 24 hydrogen atoms. In the case of Alpha-Cedrene, the molecule has a tricyclic structure.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,27 % 0,08 % 1,6 % 1,5 % 0,38 % 0,38 % 0,38 % 0,38 %0,88 % Cat.5A B C DCat.6 0,38 % 0,38 % 0,38 % 0,38 %0,88 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 3,1 % 3,1 %0,16 % 2,9 % 11 % 11 %5,8 % 5,8 %No Restriction Cat.10A BCat.11A BCat.12 11 % 11 %5,8 % 5,8 %No Restriction
-
Contributions from other sources
The natural contribution of Cedrene is determined by the sum of the natural contributions of each of its isomers.


















