Photo credits: ScenTree SAS
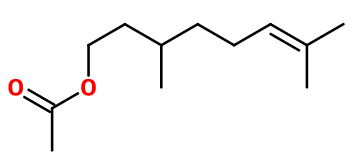
DL-citronellyl acetate
Floral > Rosy > Green Fruits > Aquatic
Citronellyl acetate ; 3,7-dimethyloct-6-enyl acetate ; acetate de 3,7-dimethyloct-6-enyl ; Citronellyl ethanoate ; Acetic acid citronellyl ester ; Citronellol acetate ; 3,7-dimethyloct-6-enol acetate ; 3,7-dimethyloct-6-enyl ethanoate ; 3,7-dimethyloct-6-enol ethanoate

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
CITRONELLYL ACETATE DRT | 900791 |
Visit website
|
Molecules |

|
- | - |
|
|
Acetate de Citronellyle - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
Citronellyl Acetate | 30035076 |
Visit website
|
Molecule | - | - | |
|
|
Citronellyl Acetate BMBcert™ | 30786719 |
Visit website
|
Molecule | - | - | |
|
|
CITRONELLYL ACETATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
150-84-5 -
EINECS number :
205-775-0 -
FEMA number :
2311 -
FLAVIS number :
09.012
-
JECFA number :
57 -
Volatility :
Head/Heart -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,891 -
Refractive Index @20°C :
1.440 - 1.450 -
Optical rotation :
Data not available. -
Vapor pressure :
0.0194 mmHg @23°C 0.0135 mmHg @25°C -
Flash Point :
93°C (199,4°F)
-
Molecular formula :
C12H22O2 -
Molecular Weight :
198,31 g/mol -
Log P :
4,22 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
240°C (464°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Citronellyl acetate is used for a fruity note in rose, lily of the valley and lavender accords for example.
Year of discovery :
Data not available.
Natural availability :
Citronellyl acetate is naturally present in Lemongrass EO, Geranium EO and Rose de Mai Absolute, and therefore is extractable in order to obtain natural Citronellyl acetate.
Isomerism :
The asymmetric carbon of Citronellol gives it two different smells if its enantiomers are separated: the (R)-(+)-Citronellyl acetate is fruity and rosy, while the (S)-(-)-Citronellyl acetate is more aldehydic, dirty and lemony. In perfumery, those two enantiomers can be used separately. In most cases, a mixture of the two is used. Menthanyl acetate, Verdox® and Vertenex® are constitutional isomers of Citronellyl acetate. However, Menthanyl acetate is much more reminiscent of Bergamot EO, and Verdox® and Vertenex® are woodier.
Synthesis precursor :
Citronellyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Citronellyl acetate can be synthesized by an esterification reaction between Citronellol and acetic acid or acetic anhydride, in an acid medium. It can also be synthesized from 3,7-dimethylocta-1,6-diene, obtained naturally by pyrolysis of alpha-Pinene. This synthesis is made in three steps: a Markovnikov addition reaction using hydrochloric acid, a Kharasch reaction, also called anti-Markovnikov reaction, with hydrobromic acid, followed by an acetolysis reaction using sodium ethanoate.
Stability :
acetates may form acetic acid through time
Other comments :
Citronellyl acetate has a less green apple note than Geranyl acetate or Neryl acetate. May contain traces of Citronellol and/or Geraniol and therefore a check must be made as it becomes a possible allergen.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,49 % 0,15 % 2,0 % 2,7 % 0,70 % 0,70 % 0,70 % 0,23 %0,82 % Cat.5A B C DCat.6 0,70 % 0,70 % 0,70 % 0,23 %0,82 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 2,4 % 2,4 %0,23 % 5,4 % 0,41 % 16 %0,23 % 0,23 %No restriction Cat.10A BCat.11A BCat.12 0,41 % 16 %0,23 % 0,23 %No restriction


















