Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
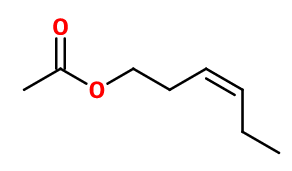
CIS-3-HEXENYL ACETATE NAT | 925002 |
Visit website
|
Molecules |

|
- | - |
|
|
Acetate de Cis-3-Hexenyle - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
CIS-3-HEXENYL ACETATE | M_0050639 |
Visit website
|
Naturel | - | - | |
|
|
CIS-3-HEXEN-1-YL ACETATE | 441H090000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - |
General Presentation
-
CAS N° :
3681-71-8 -
EINECS number :
222-960-1 -
FEMA number :
3171 -
FLAVIS number :
09.197
-
JECFA number :
134 -
Volatility :
Head/Heart -
Price Range :
€€
Physico chemical properties
-
Appearance :
Colorless liquid -
Density :
0,908 -
Refractive Index @20°C :
1,4180 - 1,4320 -
Optical rotation :
Data not available. -
Vapor pressure :
1,626 hPa @25°C -
Flash Point :
57°C
-
Molecular formula :
C8H14O2 -
Molecular Weight :
142,2 g/mol -
Log P :
2,7 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
75°C (à 26 hPa) -
Detection Threshold :
15,5193 ng/l air
Chemistry & Uses
Uses in perfumery :
cis-3-Hexenyl acetate is used in fruity-pear, apple and banana accords and in floral notes for an aqueous and airy note. Brings a green, aqueous and fruity facet at the same time.
Year of discovery :
Data not available.
Natural availability :
Cis-3-Hexenyl acetate is present in the fragrant principle of several fruits such as apple or guava and is also present in hyacinth, Sambac Jasmine Absolute and Ylang-Ylang Extra EO (and other ylang fractions), from which it can be extracted in its natural state in small quantities.
Isomerism :
Cis-3-Hexenyl acetate diastereoisomer, trans-3-Hexenyl acetate, has almost identical facets as its smell is green, fruity-pear and banana. Another position isomer, called trans-2-Hexenyl acetate, is also useful in perfumery for a less green but more fruity note, reminiscent of apple and pear. Moreover, cis-3-Hexenyl acetate is a constitutional isomer of delta-Octalactone, although they do not share the same smell and their structure are very different.
Synthesis precursor :
Cis-3-Hexenyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Cis-3-Hexenyl acetate results from an esterification of cis-3-Hexenol with acetic acid, in the presence of an acid catalysor as concentrated sulfuric acid. Using acetic anhydride or chloroacetic acid can also enhance the yield of this reaction.
Stability :
acetates may form acetic acid through time. Cis-3-Hexenyl acetate is especially unstable in shower gel and shampoo bases.
Other comments :
cis-3-Hexenyl acetate is more aqueous and greener than Hexyl acetate, with no jasmine aspect. It also is less etheric than cis-3-Hexenyl Formate.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

