Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
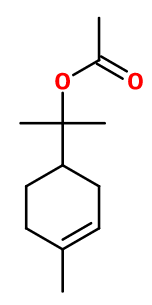
TERPINYL ACETATE | 902280 |
Visit website
|
Molecules |


|
- | - |
|
|
Terpinyl acetate | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
80-26-2 -
EINECS number :
201-265-7 -
FEMA number :
3047 -
FLAVIS number :
09.015
-
JECFA number :
368 -
Volatility :
Head -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,96 -
Refractive Index @20°C :
1,465 -
Optical rotation :
Data not available. -
Vapor pressure :
0.003 mmHg @20°C 0.038 mmHg @25°C -
Flash Point :
101°C (213,8°F)
-
Molecular formula :
C12H20O2 -
Molecular Weight :
196,29 g/mol -
Log P :
4,4 -
Fusion Point :
-20°C (-4°F) -
Boiling Point :
232°C (449,6°F) -
Detection Threshold :
2,5 ppm (0,00025%)
Chemistry & Uses
Uses in perfumery :
Terpinyl acetate is used to reproduce bergamot at a lower cost, as its smell is so close to linalyl acetate, but more associated with cheap perfumes.
Year of discovery :
Data not available.
Natural availability :
Terpinyl acetate is present in a large quantity in certain varieties of Cardamom EO, Laurel EO, Clary Sage EO and Red Thyme EO, from which it can be extracted in its natural state.
Isomerism :
Terpinyl acetate has an asymmetric carbon. The two enantiomers are not separated to be used in perfumery. Geranyl acetate, Neryl acetate, Linalyl acetate and Isobornyl acetate are isomers of Terpinyl acetate. Only Linalyl acetate has a smell of Bergamot EO, although more zesty. Geranyl acetate and Neryl acetate smell like pear and rose, while Isobornyl acetate is close to pine.
Synthesis precursor :
Terpinyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
The synthesis of Terpinyl acetate can be made by esterification of alpha-Terpineol in the presence of acetic acid or acetic anhydride, but also from alpha-Pinene which is easily extractable naturally, in two steps. Plunging alpha-Pinene in an aqueous acidic medium, converts it to 1,8-terpin with a 90% yield. When this molecule is placed in an acid medium, alpha-Terpineol and its derivatives are formed, including Terpinyl acetate. Then, it is removed from the mixture by fractional distillation.
Stability :
acetates may form acetic acid through time
Other comments :
In terms of smell, Terpinyl acetate is to be compared with Linalyl acetate and Menthanyl acetate. It brings an additional sweet facet.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















