Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
ALDAROM | 2311160710 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - |
|
|
ADOXAL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
141-13-9 -
EINECS number :
205-460-8 -
FEMA number :
4768 -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Head -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,852 -
Refractive Index @20°C :
1.450 - 1.455 -
Optical rotation :
Data not available. -
Vapor pressure :
0.003 mmHg @20°C -
Flash Point :
126°C (258,8°F)
-
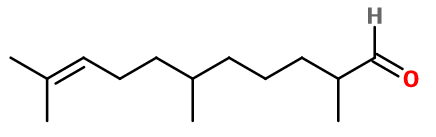
Molecular formula :
C14H26O -
Molecular Weight :
210,36 g/mol -
Log P :
>6,00 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
-
Detection Threshold :
5,38 ng/l air
Chemistry & Uses
Uses in perfumery :
Adoxal® is used to work on aqueous, ozonic and juicy fruits notes for a very similar appearance to Calone®.
Year of discovery :
Data not available.
Natural availability :
Adoxal® is not available in its natural state.
Isomerism :
Adoxal® has several asymmetric carbons. A mixture of its isomers is used in perfumery. Sandalore® is a constitutional isomer of Adoxal®. Nevertheless, this isomer has a very different smell as it is woodier and more sandalwood.
Synthesis precursor :
Adoxal® forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Synthesis route :
Adoxal® is synthesized from tetrahydroionone and alkyl chloroacetate by glycidyl ester condensation. This step is followed by a hydrolysis and a decarboxylation.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell.
Olfactively stable in any kind of products, except bleaches and other alkaline or stongly acidic detergents.
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















