Photo credits: ScenTree SAS
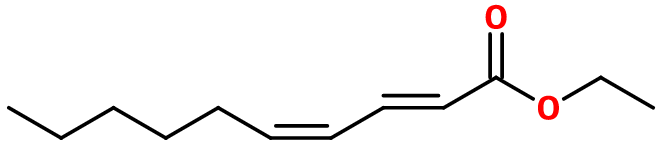
Pear Ester
Fruity > Green Fruits > Green
Ethyl 2,4-decadienoate ; Ethyl deca-2,4-dienoate ; Ethy trans-2-cis-4-decadienoate ; Ethyl deca-trans-2-cis-4-dienoate ; Ethyl (E)-2-(Z)-4-decadienoate ; Ethyl deca-(E)-2-(Z)-4-dienoate ; Ethyl decadienotate ; Ester poire ; Ethyl (E,Z)-2,4-décadienoate ; Ester de poire

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
ETHYL-2,4-DECADIENOATE | 441E180000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - |
|
|
ETHYL TRANS-2-CIS-4 DECADIENOATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
3025-30-7 -
EINECS number :
221-178-8 -
FEMA number :
3148 -
FLAVIS number :
09.260
-
JECFA number :
1192 -
Volatility :
Head/Heart -
Price Range :
€€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,91 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
113°C (235,4°F)
-
Molecular formula :
C12H20O2 -
Molecular Weight :
196,29 g/mol -
Log P :
4,61 -
Fusion Point :
< -60°C (< -76°F) -
Boiling Point :
71°C (159,8°F) -
Detection Threshold :
100 ppb (0,00001%)
Chemistry & Uses
Uses in perfumery :
Pear Ester is used in apple and pear reconstitutions, in association with violet notes (Alpha-Ionone, Beta-Ionone), with exotic fruits notes. It can also bring the fruity aspect of a rose accord.
Year of discovery :
Data not available.
Natural availability :
Pear Ester is so-called because it is found in the odorous principle of pears, and particularly in Williams pears. It is also found in apple, grapes, durian and other fruits. It does not exist on a natural state for perfumery.
Isomerism :
Pear Ester is a purely isomeric component. It is the major component formed during its synthesis, also including ethyl trans-2-trans-4-decadienoate, its diastereoisomer. It is separated from this component by fractionnal distillation. Pear Ester also is a constitutionnal isomer of numerous other esters as Linalyl acetate, Geranyl acetate and Allyl Cyclohexyl Propionate.
Synthesis precursor :
Pear Ester is not used for the synthesis of another compound used in perfumery.
Synthesis route :
Pear Ester is prepared in three steps. Starting from cis-1-heptenyl, the first step is a conversion of this molecule into 1-heptenyl lithium, in the presence of a lithium and copper iodide complex. Reacting the intermediary product with Ethyl Propiolate leads to a mixture of Pear Ester (95% of the mixture) and trans-2-trans-4-decadienoate (5% of the mixture). The last step is a fractionnal distillation to purify Pear Ester. Pear Ester is also prepared on a biotechnological way. The latter is not detailled here.
Stability :
Esters tend to form their corresponding acid in stability.
Other comments :
In comparision to a pear note as Hexyl acetate, Pear Ester brings a juicy and natural fruit facet.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















