Photo credits: ScenTree SAS
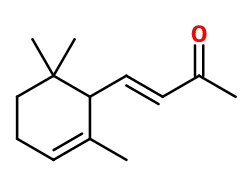
Alpha-ionone
Floral > Powdery Flowers > Violet Flower > Berries
α-Irisone® ; (E)-4-(2,6,6-trimethyl-1-cyclohex-2-enyl)but-3-en-2-one ; Alphaline 70 ; 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one ; Trans-alpha-cyclocitrylidene acetone ; Ionanthem ; (3E)-4-(2,6,6-trimethylcyclohex-2-enyl)but-3-en-2-one

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Ionone Alpha - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
IONONE ALPHA 80% | - |
Visit website
|
- | 10 grs | - | |
|
|
IRISONE ALPHA (IONONE ALPHA) | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
127-41-3 -
EINECS number :
204-841-6 -
FEMA number :
2594 -
FLAVIS number :
07.007
-
JECFA number :
388 -
Volatility :
Heart/Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,927 -
Refractive Index @20°C :
1.497 - 1.502 -
Optical rotation :
Data not available. -
Vapor pressure :
0.00097 mmHg @20°C 0.0142 mmHg @25°C -
Flash Point :
94°C (201,2°F)
-
Molecular formula :
C13H20O -
Molecular Weight :
192,3 g/mol -
Log P :
3,9 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
261°C (501,8°F) -
Detection Threshold :
0,6 à 10 ppb (0,000001%)
Chemistry & Uses
Uses in perfumery :
Alpha-Ionone is used in raspberry and floral notes of violet, orris root, rose, or carnation, for a floral-fruity and powdery facet.
Year of discovery :
This discovery was made in 1896 starting from Irones, isolated from orris butter. Ionones were discovered randomly, when a laboratory assistant added sulfuric acid to dishwash a glass containing a wrong intermediary to irone synthesis.
Natural availability :
Alpha-Ionone is present in a few rare natural ingredients. In most cases, it is synthetic alpha-Ionone that is used in perfumery.
Isomerism :
Alpha-Ionone is obtained during the cyclization step of the pseudoionone, using sulfuric acid instead of phosphoric acid, in a catalytic amount. Gamma-Ionone is obtained by using boron trifluoride as a catalyst. Alpha-Damascone® and Beta-Damascone® are positional isomers of Ionones. Their ketone function as well as a methyl group are in different parts of the molecule. Their smell is modified: Damascone® have a smell of cooked apple instead of violet.
Synthesis precursor :
alpha-Ionone is a precursor to the synthesis of Dihydro-alpha-ionone.
Synthesis route :
Nowadays, the synthesis of Ionones is made from Citral, by reaction with acetone. The category of compounds obtained is called Pseudoionone. The cyclization of Pseudoionones into Ionones is done in an acid medium. Phosphoric acid is the most preferable acid for synthesizing alpha-Ionone. The yield of this cyclization is 80% in favor of alpha-Ionone. In general, each ionone synthesis results in the formation of isomers of the desired molecule. In the case of alpha-Ionone, about 85% purity can be achieved on an industrial scale.
Stability :
Unstable in very acidic, except fabric conditioners, and very alkaline products
Other comments :
Alpha-Ionone is more floral-violet and sweeter than beta-Ionone, which is woodier. Gamma-Ionone is quite woody and resinous.
The first use of Alpha-Ionone in perfumery was in 1893, in Vera Violetta, by Roger et Gallet.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment