Photo credits: ScenTree SAS

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
3-METHYLBUTYRALDEHYDE | 441M085000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - |
|
|
ISOVALERIC ALDEHYDE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
590-86-3 -
EINECS number :
209-691-5 -
FEMA number :
2692 -
FLAVIS number :
05.006
-
JECFA number :
258 -
Volatility :
Head -
Price Range :
Data not available.
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,803 -
Refractive Index @20°C :
1,382 -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
- 5°C (23°F)
-
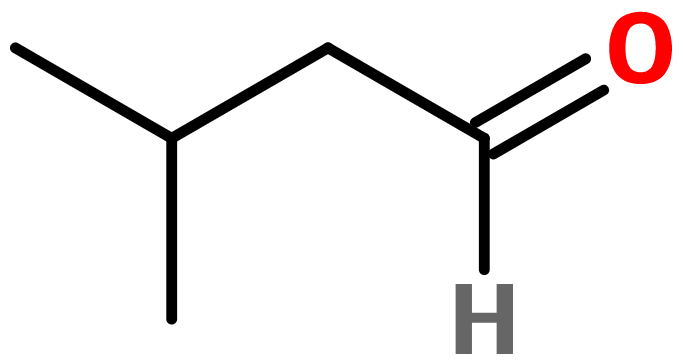
Molecular formula :
C5H10O -
Molecular Weight :
86,13 g/mol -
Log P :
1,5 -
Fusion Point :
-51°C (-59,8°F) -
Boiling Point :
90°C (194°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Isovaleraldehyde is used in cherry and chocolate notes, to bring a liquorous head note.
Year of discovery :
Data not available.
Natural availability :
Isovaleraldehyde is found in the odorous principle of many fruits and food we consume every day : apple, ginger, beef, lovage…
Isomerism :
Isovaleraldehyde does not have any isomer used in perfumery.
Synthesis precursor :
Isovaleraldehyde can be used to synthesize Schiff bases, reacting with Methyl Anthranilate or Indole for example.
Synthesis route :
Isovaleraldehyde is synthesized by oxydation of Isoamyl Alcohol.
Stability :
This molecule may form Schiff bases by reacting with Methyl Anthranilate or Indole for example, forming another colored and differently smelling compound.
Other comments :
Isovaleraldehyde can be compared to Furfural, standing out thanks to its more pyrogenic and bitter almond gourmand note. Isovaleraldehyde is more etheric and fruity-cherry and pear.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















