Photo credits: ScenTree SAS
Frambinone®
Fruity > Berries > Musky > Dry Woods
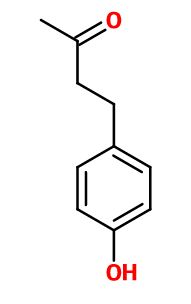
Raspberry Ketone® ; 4-(4-hydroxyphenyl)butan-2-one ; 4-(3-oxobutyl)phenol ; Para-hydrobenzyl acetone ; 1-para-hydroxyphenyl-3-butanone ; 4-(4-hydroxyphenyl)-2-butanone ; 1-(4- hydroxyphenyl)-3-butanone ; Para-hydroxyphenylbutanone ; N112 ; N 112 ; Oxanone ; Oxyphenylon ; Rasketone ; Raspberry keytone ; Rastone ; Rheosmin

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Frambinone - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
RASPBERRY KETONE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
5471-51-2 -
EINECS number :
226-806-4 -
FEMA number :
2588 -
FLAVIS number :
07.055
-
JECFA number :
728 -
Volatility :
Heart/Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
White solid -
Density :
N/A -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
0.0010 mmHg @20°C -
Flash Point :
94°C (201,2°F)
-
Molecular formula :
C10H12O2 -
Molecular Weight :
164.20 g/mol -
Log P :
0,94 -
Fusion Point :
83°C (181,4°F) -
Boiling Point :
292°C (557,6°F) -
Detection Threshold :
0,1 ppm (0,00001%)
Chemistry & Uses
Uses in perfumery :
Frambinone® brings a fruity evocation in all types of accords. Used in raspberry reconstitutions and other red fruit notes.
Year of discovery :
1918
Natural availability :
Frambinone® is present in several fruits and is extractable in its natural state, but only synthetic Frambinone® is used in perfumery.
Isomerism :
The meta and ortho positional isomers of Frambinone® are not used in perfumery. Styrallyl acetate, Eugenol and Benzyl Propionate are some of the constitutional isomers of Frambinone® that are used in perfumery. Their smell is however very different, as it is fruity-rhubarb, spicy or floral-white flowers.
Synthesis precursor :
Frambinone® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Frambinone® is synthesized in two steps. The first is a condensation between 4-hydroxybenzaldehyde and acetone, to obtain 4-hydroxybenzalacetone. A catalytic and selective hydrogenation of the double bond formed allows to obtain the final product, Frambinone®.
Stability :
Stable in perfumes and diverse functional bases
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,68 % 1 % 0,27 % 1 % 1 % 0,14 % 0,27 % 0,045 %0,82 % Cat.5A B C DCat.6 1 % 0,14 % 0,27 % 0,045 %0,82 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,41 % 0,41 %0,045 % 1 % 1 % 1 %0,045 % 0,045 %78 % Cat.10A BCat.11A BCat.12 1 % 1 %0,045 % 0,045 %78 %
-
Contributions from other sources
4-(4-Hydroxyphenyl)butan-2-one has been found in natural extracts but only at trace levels.