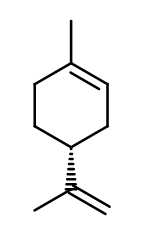
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
D-Limonene 30 Gr | - |
Visit website
|
- | - | - |
General Presentation
-
CAS N° :
5989-27-5 -
EINECS number :
227-813-5 -
FEMA number :
2633 -
FLAVIS number :
01.045
-
JECFA number :
1326 -
Volatility :
Head/Heart -
Price Range :
€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,842 -
Refractive Index @20°C :
1.471 - 1.477 -
Optical rotation :
Data not available. -
Vapor pressure :
1.43 mmHg @20°C -
Flash Point :
50°C (122°F)
-
Molecular formula :
C10H16 -
Molecular Weight :
136,23 g/mol -
Log P :
4,2 -
Fusion Point :
-74°C (-101,2°F) -
Boiling Point :
176°C (348,8°F) -
Detection Threshold :
10 ppb (0,000001%)
Chemistry & Uses
Uses in perfumery :
D-Limonene is used in reconstitutions of all types of citrus fruits, to bring head and freshness to a fragrance.
Year of discovery :
Data not available.
Natural availability :
D-Limonene is most often obtained naturally, as it is present at about 98% in Sweet Orange EO and in many other citrus fruits essential oils. Therefore, a simple distillation allows to obtain it in its natural state.
Isomerism :
D-Limonene has two enantiomers. While D-Limonene is the major component of most citrus essential oils, L-Limonene is present in a small amount in mints and conifers. The smell of L-Limonene is very terpenic and conifer-like and D-Limonene is very zesty and orange. L-Limonene is also much less produced, as it has less olfactory interest. The rotatory power of the two molecules is opposite, which explains why the racemic mixture of the two compounds is optically stable. Myrcene, Ocimene, Pinene and Terpinene are constitutional isomers of D-Limonene. All these isomers also belong to the family of molecules called terpenes, although they do not share the same smell. These molecules have in common that they can be synthesized by a Diels-Alder reaction, starting with the molecule called Isoprene.
Synthesis precursor :
D-Limonene is a precursor for the synthesis of several compounds of olfactory interest. Its oxidation allows to obtain several products. Dehydrogenation gives para-Cymene. A hydrohalogenation followed by an acid hydrolysis of the compound leads to the formation of cyclic terpene alcohols. Finally, the reaction of nitrosyl chloride with D-Limonene is a reaction used to synthesize L-Carvone.
Synthesis route :
By synthesis, a mixture of the two isomers of Limonene is obtained. D-Limonene is prepared during isomerization of alpha-Pinene and beta-Pinene by acid catalysis. The distillation of the so-called ''dipentene '' fraction, obtained after this isomerization, allows to obtain D-Limonene and L-Limonene separately, with different purity degrees.
Stability :
May form L-Carvone through time in alcoholic perfumes.
Terpenes tend to polymerize by oxydation.
Very unstable in very alkaline bases as soap, in which its smell is not percievable.
Other comments :
D-Limonene is one of the 26 allergens in perfumery.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
-
Specified ingredients: notes
Oxidation products of Limonene, especially hydroperoxides, have been demonstrated to be potent sensitizers. d-, l- and dl-Limonene and natural products containing substantial amounts of it, should only be used when the level of (hydro)peroxides is kept to the lowest practical level, for instance by adding antioxidants at the time of production. The addition of 0.1% BHT or α-Tocopherol for example has shown great efficiency. Such products should have a peroxide value of less than 20 millimoles per liter, determined according to the IFRA analytical method for the determination of the peroxide value, which can be downloaded from the IFRA website (www.ifrafragrance.org).