Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
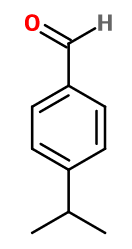
CUMINIC ALDEHYDE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
122-03-2 -
EINECS number :
204-516-9 -
FEMA number :
2341 -
FLAVIS number :
05.022
-
JECFA number :
868 -
Volatility :
Head -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,985 -
Refractive Index @20°C :
1.529 - 1.534 -
Optical rotation :
Data not available. -
Vapor pressure :
0.0697 mmHg @20°C 0.048 mHg @25°C -
Flash Point :
94°C (201,2°F)
-
Molecular formula :
C10H12O -
Molecular Weight :
148,2 g/mol -
Log P :
2,98 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
235°C (455°F) -
Detection Threshold :
34,1731 ng/l air
Chemistry & Uses
Uses in perfumery :
Cuminaldehyde is used in leather, spicy notes, in heavy and spicy flower accords (e. g. carnation), in oriental and woody accords and for sweaty notes. Used as a replacor for Cumin EO, in which it is found at about 30% of the composition.
Year of discovery :
Data not available.
Natural availability :
Cuminaldehyde can be extracted in its natural state from Cumin EO, as it represents 40% of its composition.
Isomerism :
The ortho and meta isomers isolated from Cuminaldehyde are not used in fine perfumery. Cuminaldehyde is a constitutional isomer of Anethole, which has a very different smell reminiscent of Star Anise EO.
Synthesis precursor :
Cuminaldehyde is the precursor to several raw materials. Its oxidation gives Cuminic Acid, the hydrogenation of the aldehyde function leads to Cuminic Alcohol and its total hydrogenation synthesizes Mayol with a smell of lily of the valley. It can also form a Schiff base with Methyl Anthranilate, with a fruity and more artificial smell, or a dimethyl acetal which also has an olfactory interest.
Synthesis route :
Cuminaldehyde synthesis is made by reduction of 4-Isopropylbenzoyl chloride or by formylation of cumene.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,085 % 0,025 % 0,51 % 0,47 % 0,12 % 0,12 % 0,12 % 0,12 %0,28 % Cat.5A B C DCat.6 0,12 % 0,12 % 0,12 % 0,12 %0,28 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,96 % 0,96 %0,05 % 0,92 % 3,3 % 3,3 %1,8 % 1,8 %No Restriction Cat.10A BCat.11A BCat.12 3,3 % 3,3 %1,8 % 1,8 %No Restriction


















