Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
ALLYL HEXANOATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
123-68-2 -
EINECS number :
204-642-4 -
FEMA number :
2032 -
FLAVIS number :
09.244
-
JECFA number :
3 -
Volatility :
Head -
Price Range :
€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,889 -
Refractive Index @20°C :
1.422 - 1.426 -
Optical rotation :
Data not available. -
Vapor pressure :
0.678 mmHg @25°C -
Flash Point :
74°C (165,2°F)
-
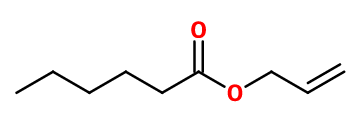
Molecular formula :
C9H16O2 -
Molecular Weight :
156,23 g/mol -
Log P :
3,2 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
190°C (374°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Allyl Caproate is used in pineapple and apple notes, for a ripe and sweet effect. Also used in exotic and yellow fruit notes.
Year of discovery :
Data not available.
Natural availability :
Allyl Caproate is present in pineapple and mushroom. There is also an Allyl Caproate of natural origin.
Isomerism :
Isoamyl Butyrate and gamma-Nonalactone are constitutional isomers of Allyl Caproate. Isoamyl Butyrate also has a fruity and butyric smell, but gamma-Nonalactone has a very different note of peach and coconut.
Synthesis precursor :
Allyl Caproate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Allyl Caproate is prepared by esterification between Hexanoic acid and allylic alcohol (or prop-2-en-1-ol), by acid catalysis.
Stability :
May form caproic acid through time
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















