Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
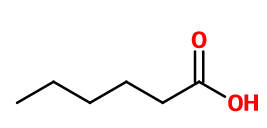
HEXANOIC ACID (CAPROIC) | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
142-62-1 -
EINECS number :
205-550-7 -
FEMA number :
2559 -
FLAVIS number :
08.009
-
JECFA number :
93 -
Volatility :
Heart/Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Oily colorless liquid -
Density :
0,925 -
Refractive Index @20°C :
1.415 - 1.418 -
Optical rotation :
Data not available. -
Vapor pressure :
0.0435 mmHg @25°C -
Flash Point :
102°C (215,6°F)
-
Molecular formula :
C6H12O2 -
Molecular Weight :
116,16 g/mol -
Log P :
1,75 -
Fusion Point :
-3°C (26,6°F) -
Boiling Point :
205°C (401°F) -
Detection Threshold :
93 ppb à 100 ppm
Chemistry & Uses
Uses in perfumery :
Caproic Acid is used in very small quantity in cheese and lavender notes.
Year of discovery :
Data not available.
Natural availability :
Caproic Acid can be obtained naturally, as it is present in many plant extracts, such as Lavender EO, Petitgrain EO or Lemongrass EO. Therefore, it can be extracted from these essential oils by fractional distillation.
Isomerism :
Ethyl Butyrate is a constitutional isomer of Caproic Acid. Indeed, Ethyl Butyrate has a very butyric and cheesy note.
Synthesis precursor :
Caproic acid is the precursor for the synthesis of several esters called ''caproates '', by reacting it with an alcohol, in the presence of an acid catalyst.
Synthesis route :
Caproic acid is synthesized from chloropentane by a two-step reaction, by reaction with sodium cyanide, followed by hydrolysis.
Stability :
Stable in perfumes and diverse functional bases
Les acides font baisser le pH d'un produit parfumé. Un pH acide peut mieux conserver le parfum, comme être la sources de modification d'autres matières premières.
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















