Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
CIS-6-NONENAL WITH ATP | 441N020000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - |
|
|
CIS-6-NONENAL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
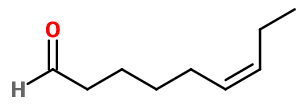
CAS N° :
2277-19-2 -
EINECS number :
218-900-9 -
FEMA number :
3580 -
FLAVIS number :
05.059
-
JECFA number :
325 -
Volatility :
Head -
Price Range :
€€€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,841 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
113°C (235,4°F)
-
Molecular formula :
C9H16O -
Molecular Weight :
140,23 g/mol -
Log P :
Donnée indisponible. -
Fusion Point :
Donnée indisponible. -
Boiling Point :
87°C (188,6°F) -
Detection Threshold :
De l'ordre du ppb, 10 millionièmes de pourcent
Chemistry & Uses
Uses in perfumery :
Cis-6-Nonenal is used in reproductions of fruity notes of cucumber, melon, and to bring an aqueous note to fruity accords and perfumes. Also used in violet leaf notes, to provide an aqueous facet.
Year of discovery :
Data not available.
Natural availability :
Cis-6-Nonenal is one of the main components of the odorous principle of melon and cucumber. It is also found in some fish and in a fruit called pepino dulce.
Isomerism :
The trans diastereoisomer of cis-6-Nonenal is much less used in perfumery. Melonal® is also a position isomer of cis-6-Nonenal. The main chain of Melonal® is shorter, and has two ramifications. The result is a fruity smell, closer to melon, but with similar aqueous accents.
Synthesis precursor :
Cis-6-nonenal, like all aldehydes, can be used to synthesize Schiff bases, by reaction with Methyl Anthranilate or Indole for example. These reactions often lead to the formation of a very powerful and coloured product.
Synthesis route :
Cis-6-Nonenal is synthesized by a catalytic hydrogenation reaction (e. g. platinum catalysis) of 6-nonynal diethyl acetal. The latter is obtained by reaction between sodium ethanolate (obtained by reaction between pure sodium and ethanol) and the latter alkyne. The product obtained as a result of this first synthesis step is then subjected to an acid hydrolysis to obtain the final synthesis product: cis-6-Nonenal.
Stability :
Aldehyde can form their diethylacetal in alcoholic stability, bringing not so much olfactive change, but less efficiency of the ingredient.
Other comments :
Cis-6-Nonenal, like trans-2-cis-6-Nonadienal, is a very powerful note, with a very low detection threshold. It must therefore be used in dilution in the compositions. In addition, it is more used for melon notes than trans-2-cis-6-Nonadienal, which is more reminiscent of cucumber.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















