Photo credits: ScenTree SAS
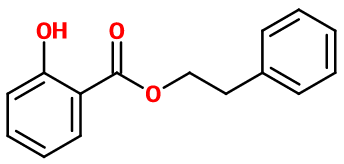
Phenylethyl salicylate
Floral > Light Flowers > Rosy
Benzoate de 2-hydroxy-2-phenylethyl ; 2-hydroxy-2-phenylethyl benzoate ; 2-hydroxybenzoate de benzylcarbinyl ; Benzylcarbinyl 2-hydroxybenzoate ; Benzyl carbinyl salicylate ; Salicylate de benzylcarbinyl ; Salicylate de phenethyl ; Phenethyl salicylate ; Diethyl-1,2-dihydroxy-1,2-ethanedicarboxylate ; 1,2-dihydroxy-1,2-ethanedicarboxylate de diethyl

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
PHENYLETHYL SALICYLATE | 368 |
Visit website
|
Molecule | - | - | |
|
|
PHENYLETHYL SALICYLATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
87-22-9 -
EINECS number :
201-732-5 -
FEMA number :
2868 -
FLAVIS number :
09.753
-
JECFA number :
905 -
Volatility :
Heart/Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
White solid -
Density :
1,15 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
113°C (235,4°F)
-
Molecular formula :
C15H14O3 -
Molecular Weight :
242,27 g/mol -
Log P :
Donnée indisponible. -
Fusion Point :
41°C (105,8°F) -
Boiling Point :
370°C (698°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Phenyl Ethyl Salicylate is sometimes used as a base note in floral bouquets, spicy-floral and balsamic perfumes, to make the link between a white flower and a rosy note for example. It brings volume, tenacity and a balsamic effect to a composition.
Year of discovery :
Data not available.
Natural availability :
No natural extract include Phenyl Ethyl Salicylate among their major components.
Isomerism :
Phenyl Ethyl Salicylate does not have any isomer used in perfumery.
Synthesis precursor :
Phenyl Ethyl Salicylate is not used for the synthesis of another compound used in perfumes.
Synthesis route :
Phenyl Ethyl Salicylate can be synthesized in two ways. The first one is an interchange reaction involving Phenyl Ethyl Alcohol and Methyl Salicylate. The second synthetic route is an esterification reaction between Salicylic Acid and Phenyl Ethyl Alcohol. This reaction involves an acidic catalysis to raise its yield.
Stability :
My form Salicylic acid in stability.
Most of the time, the presence of an aromatic cycle in the molecule brings coloration through time.
Unstable in acidic products, except antiperspirants, and in very alkaline products.
Other comments :
Phenyl Ethyl Salicylate is the only solid compound among other salicylates used in perfumes. On the other hand, it is the most rosy one. In comparision, Cis-3-Hexenyl Salicylate is used in green white flowers notes, and Benzyl Salicylate is used in jasmine notes for example.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















