Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
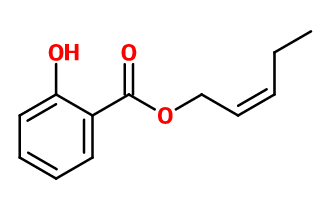
CIS 3 HEXENYL SALICYLATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
65405-77-8 -
EINECS number :
265-745-8 -
FEMA number :
4750 -
FLAVIS number :
09.570
-
JECFA number :
Donnée indisponible. -
Volatility :
Heart/Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
1,061 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
0.00033 mmHg @23°C -
Flash Point :
94°C (201,2°F)
-
Molecular formula :
C13H16O3 -
Molecular Weight :
220,27 g/mol -
Log P :
4,36 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
-
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Cis-3-Hexenyl Salicylate is used for solar, ylang-ylang and tuberose notes and for fougere perfumes. Brings a green note to white flowers. Provides a good binding between top and bottom notes.
Year of discovery :
Data not available.
Natural availability :
Cis-3-Hexenyl Salicylate is present in a small amount in carnation absolute. Knowing that carnation offers only a very low extraction yields, natural cis-3-Hexenyl Salicylate is very little produced. The synthetic compound is more often used in perfumery.
Isomerism :
Trans-3-Hexenyl Salicylate, a diastereoisomer of cis-3-Hexenyl Salicylate, exists but is very little used.
Synthesis precursor :
Cis-3-Hexenyl salicylate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
As for other Salicylates, cis-3-Hexenyl Salicylate is synthesized by an esterification reaction between salicylic acid and cis-3-hexenol. It is the use of cis-3-Hexenol which gives the molecule its characteristic green note of cut grass.
Stability :
May form Salicylic acid through time.
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Other comments :
Comparing it to other salicylates, cis-3-Hexenyl Salicylate is the greenest.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















