Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
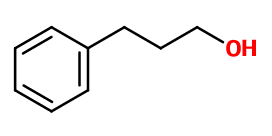
PHENYLPROPYL ALCOHOL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
122-97-4 -
EINECS number :
204-587-6 -
FEMA number :
2885 -
FLAVIS number :
02.031
-
JECFA number :
636 -
Volatility :
Heart -
Price Range :
€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,999 -
Refractive Index @20°C :
1.524 - 1.528 -
Optical rotation :
Data not available. -
Vapor pressure :
0.003 mmHg @25°C -
Flash Point :
112°C (233,6°F)
-
Molecular formula :
C9H12O -
Molecular Weight :
136,19 g/mol -
Log P :
1,9 -
Fusion Point :
-18°C (-0,39°F) -
Boiling Point :
235°C (455°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Phenyl Propyl Alcohol can be used to create ambery and rosy accords. Interesting to give nuance to warm spices or woods. Can bring warmth to floral notes such as carnation, jasmine, narcissus, lilac...
Year of discovery :
Data not available.
Natural availability :
Phenyl Propyl Alcohol extraction is possible from Siam Benzoin Resinoid (and other origins), Peru Balsam Resinoid or Ceylon Cinnamon EO (and other origins).
Isomerism :
Phenyl Propyl Alcohol positional isomer, 2-Phenylpropanol, has a more green and spicy smell. The other isomer, 1-Phenylpropanol is less cinnamic and more floral.
Synthesis precursor :
Phenyl Propyl Alcohol is a precursor to the synthesis of Phenyl Propyl Aldehyde by reduction and Phenyl Propyl Acid by oxidation. Its esters also have an olfactory importance.
Synthesis route :
Its synthesis can be done in two ways. The first is a catalytic hydrogenation of Cinnamaldehyde, obtained by synthesis or from Cassia EO. An oxo synthesis modified from styrene causes the formation of the Phenylpropyl Alcohol, but also of its positional isomer, 2-Phenylpropanol. The two isomers can be separated by a fractional distillation.
Stability :
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Other comments :
Phenyl Propyl Alcohol is more honeyed, warm and cinnamic than Phenyl Ethyl Alcohol.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment