Photo credits: ScenTree SAS

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
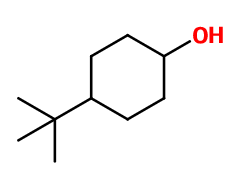
PTBC HEXANOL | PATCH-1 |
Visit website
|
- | 10 grs | 98.5 - 100 |
General Presentation
-
CAS N° :
98-52-2 -
EINECS number :
202-676-4 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Heart/Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
White solid -
Density :
2,591 -
Refractive Index @20°C :
Donnée indisponible. -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
Donnée indisponible. -
Flash Point :
105°C (221°F)
-
Molecular formula :
C10H20O -
Molecular Weight :
156,27 g/mol -
Log P :
3,31 -
Fusion Point :
66°C (150,8°F) -
Boiling Point :
113°C (235,4°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Patchone® is used in coniferous, patchouli, rose, violet notes to dry some floral notes. Also used in tea accords, patchouli reconstitutions, spring flowers notes and citrus-grapefruit notes.
Year of discovery :
1939
Natural availability :
Patchone® is not available in its natural state.
Isomerism :
Rosalva®, L-Menthol and Citronellol are among the constitutional isomers of Patchone®. Their smell is however very different, as they are not reminiscent of Patchouli EO.
Synthesis precursor :
Patchone® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Patchone® can be synthesized by a high temperature catalytic hydrogenation of para-tertbutylphenol (obtained by a Friedel-Crafts reaction on tertbutylbenzene). As the benzene ring of this molecule is stable, a high temperature is required .
Stability :
Stable in perfumes and diverse functional bases
Other comments :
Data not available.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















