Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
MUSCONE | 962195 |
Visit website
|
Molecules |

|
- | - |
|
|
MUSCONE LAEVO | 954117 |
Visit website
|
Molecules |

|
- | - |
|
|
Muscone - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
MUSCONE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
541-91-3 -
EINECS number :
208-795-8 -
FEMA number :
3434 -
FLAVIS number :
07.111
-
JECFA number :
1402 -
Volatility :
Base -
Price Range :
€€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,922 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
0.000095 mmHg @23°C -
Flash Point :
>100°C (>212°F)
-
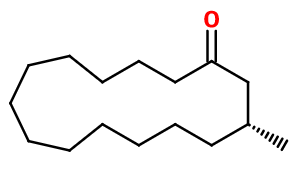
Molecular formula :
C16H30O -
Molecular Weight :
238,4 g/mol -
Log P :
6,6 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
130°C (266°F) -
Detection Threshold :
9,8 ppb (0,00000098%)
Chemistry & Uses
Uses in perfumery :
Muscone® is used in fine fragrance for musky, floral and animalic notes and in reproductions of natural musk.
Gives an interesting powdery-violet effect in leather and woody perfumes.
Year of discovery :
Discovered in 1905 but the molecular structure was first identified in 1925.
Natural availability :
Muscone® was originally found in natural musk, extracted from the Tibetan goat. Nowadays, only synthetic Muscone® is produced.
Isomerism :
This compound has an asymmetric carbon that gives rise to two possible enantiomers with a similar smell. There is also a molecule called Nor-Muscone® (or Exaltone), which has no branching. Its smell is roughly equivalent to classic Muscone®.
Synthesis precursor :
Muscone® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Muscone® is a macrocyclic musk that can be synthesized in many ways. One of them is from Citronellal to synthesize diacetyldodecane, which is the widespread method. This compound is cyclized by a ruthenium catalysis, followed by a catalytic hydrogenation to obtain the final Muscone®.
Stability :
Musks are very stable, as in alcoholic and in functional fragrances
Other comments :
Muscone® is less powerful than Muscenone®, with a similar structure.
Muscone® is the first non-nitrated musk to appear on the market. Initially extracted from Musk Tonkin (some qualities of which contain up to 20%). It took 20 years for chemists to succeed in unlocking the secret of its molecular structure. Indeed, at the beginning of the 20th century it was difficult to imagine that a molecule could have more than 6 carbons in its cycle. It was therefore necessary to wait for the arrival of more advanced analytical techniques to understand and assert its structure.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment