Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
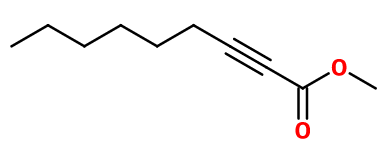
METHYL OCTINE CARBONATE | OCM-1 |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
111-80-8 -
EINECS number :
203-909-2 -
FEMA number :
2726 -
FLAVIS number :
09.156
-
JECFA number :
1356 -
Volatility :
Heart -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,921 -
Refractive Index @20°C :
Donnée indisponible. -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
Donnée indisponible. -
Flash Point :
101°C (213,8°F)
-
Molecular formula :
C10H16O2 -
Molecular Weight :
168,24 g/mol -
Log P :
4 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
122°C (251,6°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Methyl Octine Carbonate is used in notes of violet flower, tuberose, linden and mimosa. A green facet with fruity notes. Used in combination with green notes and ionones.
Year of discovery :
1903
Natural availability :
Methyl Octine Carbonate is not available in its natural state.
Isomerism :
Jasmolactone is a constitutional isomer of Methyl Octine Carbonate. Its smell is however very different, as it is reminiscent of peach and jasmine.
Synthesis precursor :
Methyl Octine Carbonate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Methyl Octine Carbonate is synthesized by an esterification reaction between non-2-ynoic acid and methanol. This reaction is catalysed by the presence of a strong concentrated acid such as sulfuric acid.
Stability :
May form octine carbonic acid through time, under the effect of heat.
Unstable in acidic products, except antiperspirants, and very alkaline products
Other comments :
In comparision to other green notes of Violet Leaf Absolute as Folione® or Violiff®, Methyl Octine Carbonate has a clear mushroom note.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,0018 % 0,00055 % 0,011 % 0,01 % 0,0026 % 0,0026 % 0,0026 % 0,0026 %Cat.5A B C DCat.6 0,0026 % 0,0026 % 0,0026 % 0,0026 %0,0061 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,021 % 0,021 %0,0011 % 0,02 % 0,072 % 0,072 %0,04 % 0,04 %Cat.10A BCat.11A BCat.12 0,072 % 0,072 %0,04 % 0,04 %No Restriction
-
Restricted ingredients: notes
When used in the same fragrance compound within a specific QRA category, the sum total of and Methyl heptine carbonate (MHC, CAS number 111-12-6) and Methyl octine carbonate (MOC, CAS number 111-80-8) contributions must not exceed the maximum permitted level for MHC. At the same time, the contribution from MOC should always respect the maximum levels permitted as listed in the table above.


















