Photo credits: ScenTree SAS
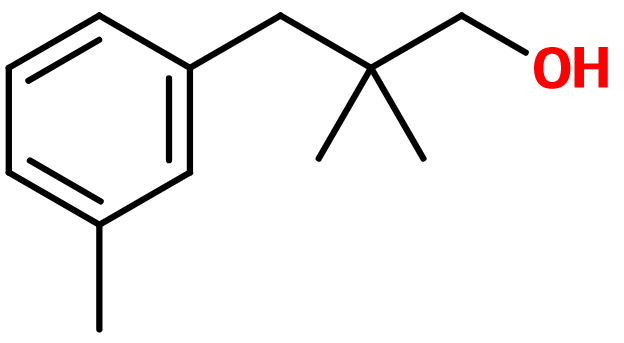
Majantol®
Floral > Light Flowers > Aldehydes > Fresh Flowers > Aquatic
2,2-dimethyl-3-(3-methylphenyl) propanol ; 2,2-dimethyl-3-(3-methylphenyl) propan-1-ol ; 3-(2,2-dimethyl-3-hydroxypropyl)toluol ; Lanjantol ; Lilivol ; Lily propanol ; Linlan alcohol ; Muguenol ; Trimethyl benzene propanol ; Trimethylbenzenepropanol

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Majantol® - 30gr | - |
Visit website
|
- | - | - | |
|
|
MAJALOL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
103694-68-4 -
EINECS number :
403-140-4 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Heart -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid that solidifies at room temperature -
Density :
0,97 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
93°C (199,4°F)
-
Molecular formula :
C12H18O -
Molecular Weight :
178,27 g/mol -
Log P :
3,38 -
Fusion Point :
24°C (75,2°F) -
Boiling Point :
289°C (552,2°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Majantol® enters the floral-aldehydic molecules category, including Lilial® and Bourgeonal™ for example. It is used as it is slightly less regulated, for lily of the valley and other white flower notes, for a light effect, in association with other molecules as Hedione® or Florol®. It also brings a fresh nuance.
Year of discovery :
Patent N°3,531,585 (DE) published on Sept. 4, 1985 by Elektrochemisch Industrie GmbH
Natural availability :
Majantol® is not reported as found in nature, and can thus not be extracted from any plant.
Isomerism :
Majantol® has a methyl group associated to carbon n°3 of its aromatic cycle, on a meta position. Molecules having this groupment on an ortho or para position are not used in perfumery, although they are isomers of Majantol® Phenoxanol® is a constitutional isomer of Majantol®, having a more rosy and green note.
Synthesis precursor :
Majantol® is not a precursor for the synthesis of another material used in perfumery.
Synthesis route :
Majantol® can be prepared in two steps, starting with 3-methylbenzyl chloride, reacting it with 2-methylpropanal, in the presence of a catalysor as tetrabutylammonium iodide. An aldehyde is obtained and can be reducted, reacting with sodium tetrahydruroborate, to obtain the final product.
Stability :
Aromatic compounds are chromophorous. This means that they may color through time and in contact with alkaline bases.
Other comments :
Majantol® structure is close to Dimethyl Benzyl Carbinol. Its smell is less aldehydic and aqueous. Majantol® also has olfactive similarities with Lyal® and Hydroxycitronellal®, although it is more aqueous than theses molecules.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,034 % 0,2 % 0,025 % 1,7 % 0,43 % 0,061 % 0,039 % 0,013 %0,0025 % Cat.5A B C DCat.6 0,43 % 0,061 % 0,039 % 0,013 %0,0025 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,052 % 0,052 %0,013 % 0,14 % 0,14 % 0,3 %0,013 % 0,013 %8,6 % Cat.10A BCat.11A BCat.12 0,14 % 0,3 %0,013 % 0,013 %8,6 %
-
Specified ingredients: notes
2,2-Dimethyl-3-(3-tolyl)propan-1-ol should only be used as a fragrance ingredient if traces of organochlorine compounds are restricted. Total Chlorine, which can be measured by Atomic Absorption Spectroscopy, must not exceed 25 ppm in the raw material.


















