Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Javanol® Super - 30gr | - |
Visit website
|
- | - | - |
General Presentation
-
CAS N° :
198404-98-7 -
EINECS number :
427-900-1 -
FEMA number :
4776 -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
2254 -
Volatility :
Base -
Price Range :
€€€€
Physico-chemical properties
-
Appearance :
Colorless viscous liquid -
Density :
0,95 -
Refractive Index @20°C :
Donnée indisponible. -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
Donnée indisponible. -
Flash Point :
> 93°C (> 199,4°F)
-
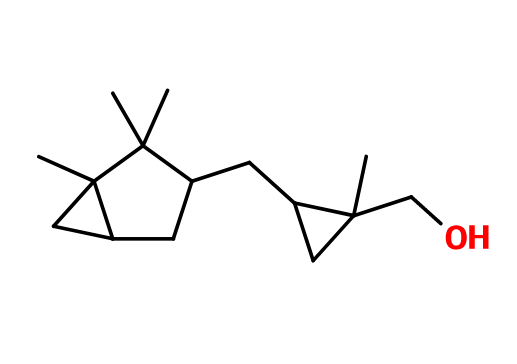
Molecular formula :
C15H26O -
Molecular Weight :
222,37 g/mol -
Log P :
Donnée indisponible. -
Fusion Point :
Donnée indisponible. -
Boiling Point :
271°C (519,8°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Javanol® is used for the same reasons as Bacdanol®, and for its aromatic facet, similar to thyme. Often used in small quantities as it is a very powerful and effective ingredient.
Year of discovery :
Data not available.
Natural availability :
Javanol® is not available in its natural state.
Isomerism :
Javanol® has several asymmetric carbons that give rise to several possible isomers. Javanol® Super is a unique isomer. In addition, Javanol® is a constitutional isomer of Polysantol®. These two compounds have a similar structure, and are used for similar reasons in perfumes. Javanol® still has a less milky note than Polysantol®, but is more powerful.
Synthesis precursor :
Javanol® is not involved in the synthesis of another compound of olfactory interest.
Synthesis route :
Javanol® is synthesized by a Simmons-Smith double reaction, consisting of cyclopropanation from 2-methyl-4-(2,2,3-trimethyl-3-cyclopenten-1-yl)-2-buten-1-ol. This reaction involves a catalysis of Zinc and Copper, as well as diodomethane, to convert a double bond to a cyclopropane group. Javanol® Super corresponds to one isolated isomer of Javanol®.
Stability :
Unstable exclusively in acid cleaners and in bleach.
Other comments :
The detection limit of Javanol® is extremely low. This makes it one of the most used santal compounds, because it brings a milky and woody effect, even with a very small dosis.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment