Photo credits: ScenTree SAS
Jasmonal A®
Floral > Light Flowers > White Flowers > Berries > Anisic
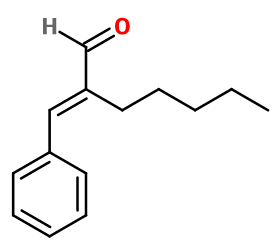
Amyl Cinnamaldehyde ; Amyl Cinnamaldehyde ; 2-benzylideneheptanal ; Amyl cinnamal ; Amylcinnamaldehyde ; Amylcinnamal ; 2-benzylidene heptanal ; Buxine ; Flomine ; Flosal ; Floxine ; 2-(phenylmethylene)-heptanal ; Jasmin aldehyde ; Jasminal ; Jasminaldehyde ; Jasmona ; Jasmine aldehyde ; 2-pentyl cinnamaldehyde

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
ALPHA AMYL CINNAMIC ALDEHYDE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
122-40-7 -
EINECS number :
204-541-5 -
FEMA number :
2061 -
FLAVIS number :
05.040
-
JECFA number :
685 -
Volatility :
Heart/Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,967 -
Refractive Index @20°C :
1.554 - 1.562 -
Optical rotation :
Data not available. -
Vapor pressure :
0.00051 mmHg @25°C -
Flash Point :
94°C (201,2°F)
-
Molecular formula :
C14H18O -
Molecular Weight :
202,3 g/mol -
Log P :
4,7 -
Fusion Point :
< 0°C (< 32°F) -
Boiling Point :
289°C (552,2°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Jasmonal A® is useful for light floral accords such as spring and white flowers : jasmine, lilac, tuberose or narcissus notes. Also used in fruity notes.
Year of discovery :
1926
Natural availability :
Jasmonal A® is found in trace amounts in some black teas. However, it is synthetic Jasmonal A® that is most often used in perfumery.
Isomerism :
Jasmonal A® has a double bond that gives rise to two possible diastereoisomers of the molecule. However, it is the mixture of the two diastereoisomers that is used in perfumery.
Synthesis precursor :
Jasmonal A® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
As for Cinnamaldehyde, the synthesis of Jasmonal A® is made by a condensation of Benzaldehyde with heptanal (Aldehyde C-7), using an excess of Benzaldehyde and gradually adding the Aldehyde C-7 in order to avoid it self-condensation.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell.
Jamsonal A colors through time.
Other comments :
Jasmonal A® is more powerful, rising and soapy, but less watery than Jasmonal H®.
Jasmonal A® is one of the 26 allergens in perfumery.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,58 % 0,53 % 0,26 % 7 % 2,5 % 0,32 % 0,45 % 0,11 %0,064 % Cat.5A B C DCat.6 2,5 % 0,32 % 0,45 % 0,11 %0,064 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,26 % 0,26 %0,11 % 1,5 % 1,5 % 3,5 %0,11 % 0,11 %No Restriction Cat.10A BCat.11A BCat.12 1,5 % 3,5 %0,11 % 0,11 %No Restriction


















