Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
ACETAL CD | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
29895-73-6 -
EINECS number :
249-934-2 -
FEMA number :
2877 -
FLAVIS number :
06.007
-
JECFA number :
1004 -
Volatility :
Head/Heart -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
1,162 -
Refractive Index @20°C :
1.529 - 1.534 -
Optical rotation :
Data not available. -
Vapor pressure :
0.00127 mmHg @20°C 0.00004 mmHg @23°C -
Flash Point :
100°C (212°F)
-
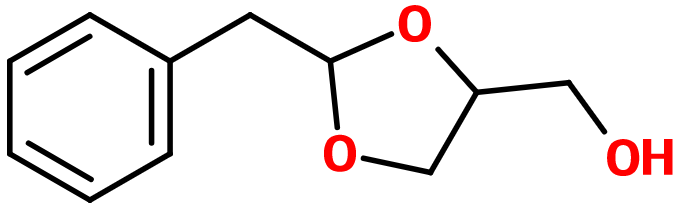
Molecular formula :
C11H14O3 -
Molecular Weight :
194,23 g/mol -
Log P :
0,8 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
358°C (676,4°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Hyacinth Acetal is used in floral-lily of the valley, rosy, spicy and marine notes in small quantities. Used in all types of perfumes.
Year of discovery :
Data not available.
Natural availability :
Hyacinth Acetal is not available in its natural state.
Isomerism :
Hyacinth Acetal has an asymmetric carbon, but it is its racemic mixture that is used in perfumery.
Synthesis precursor :
Hyacinth Acetal is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Hyacinth Acetal is synthesized by acetalization of Phenyl Acetaldehyde, by reaction of this molecule with glycerine. Hyacinth Acetal has a fruty and slightly less rosy smell than its starting reagent.
Stability :
Stable in perfumes and diverse functional bases, except very alkaline detergents and bleaches.
Other comments :
Hyacinth Acetal derivating from Phenyl Acetaldehyde, its smell remains close to it, but has a cinnamic undernote.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















