Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
DIHYDROIONONE BETA | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
17283-81-7 -
EINECS number :
241-318-1 -
FEMA number :
3626 -
FLAVIS number :
07.131
-
JECFA number :
394 -
Volatility :
Heart -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,925 -
Refractive Index @20°C :
1.479 - 1.485 -
Optical rotation :
Data not available. -
Vapor pressure :
0.00697 mmHg @20°C 0.0102 mmHg @25°C -
Flash Point :
110°C (230°F)
-
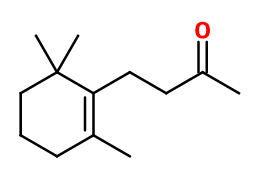
Molecular formula :
C13H22O -
Molecular Weight :
194,32 g/mol -
Log P :
4,5 -
Fusion Point :
311°C (591,8°F) -
Boiling Point :
100°C (212°F) -
Detection Threshold :
3,2772 ng/l air
Chemistry & Uses
Uses in perfumery :
DihydroBeta-Ionone is used in current and fine fragrance, in leather, rosy, red fruits, woody and lily of the valley notes.
Year of discovery :
Data not available.
Natural availability :
DihydroBeta-Ionone is present in several plant extracts, and in particular Champaca Absolute. However, only the synthetic version of this molecule is used.
Isomerism :
DihydroBeta-Ionone is a constitutional isomer of Ambrinol, also prepared with Beta-Ionone, but having a very distinctive animalic smell.
Synthesis precursor :
DihydroBeta-Ionone is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
DihydroBeta-Ionone is synthesized from beta-ionone by a catalytic hydrogenation reaction. This reaction uses a palladium, nickel or platinum catalyst. beta-Ionone, like the other ionones, is synthesized from Citral by a cyclization reaction in the presence of acetone.
Stability :
Stable in perfumes and diverse functional bases, except acid cleaners and very alkaline detergents and bleaches.
Other comments :
The smell of DihydroBeta-Ionone is much more ambery than conventional ionones. It still keeps a note of violet flower, woodier.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















