Photo credits: ScenTree SAS
Anther®
Fruity > Tropical Fruits > Lactonic > Lavender > Butyric
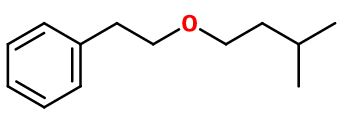
Antherex® ; Pommerol® ; 2-(3-methylbutoxy)ethylbenzene ; Isoamyl phenethyl ether ; Isoamyl phenyl ethyl ether ; Florether ; Green ether ; 3-methyl butyl oxyethyl benzene ; 3-methylbutyl 2-phenylethyl ether ; Isopentyl phenethyl ether ; Phenethyl isoamyl ether ; Phenylethyl isoamyl ether ; Treflon

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
PHENYLETHYLISOAMYL ETHER | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
56011-02-0 -
EINECS number :
259-943-3 -
FEMA number :
4635 -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
2136 -
Volatility :
Head -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,903 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
> 93°C (> 199,4°F)
-
Molecular formula :
C13H20O -
Molecular Weight :
192,3 g/mol -
Log P :
Donnée indisponible. -
Fusion Point :
Donnée indisponible. -
Boiling Point :
110°C (230°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Anther® is usually used in fruity notes to bring out a floral and aromatic effect, close to lavander.
Year of discovery :
Data not available.
Natural availability :
Anther® is not found in nature.
Isomerism :
Anther® is a constitutional isomer of alpha-Damascone® and beta-Damascone® and alpha-Ionone and beta-Ionone. Nevertheless, it doesn't have the same smell as these molecules.
Synthesis precursor :
Anther® is not a precursor to the synthesis of any other molecule used in perfumery.
Synthesis route :
Anther® can be synthesized from Phenyl Ethyl Alcohol, using a Williamson synthesis. This reaction consists in ionizing the alcohol by subjecting it to the action of a reducing metal as pure sodium or potassium. Following this first step, adding a haloalkane to the reaction mixture, here halo-3-methylbutane, transforms the alcoholate and the alcane into an ether.
Stability :
Stable in perfumes and in diverse functional bases.
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















