Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Ambrettolide - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
AMBRETTOLIDE | M_0040009 |
Visit website
|
Synthétique | - | - | |
|
|
SCENTOLIDE | 85705 |
Visit website
|
Molecule | - | - | |
|
|
AMBRETTOLIDE | AMBR-1 |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
28645-51-4 -
EINECS number :
249-120-7 -
FEMA number :
4145 -
FLAVIS number :
10.063
-
JECFA number :
1991 -
Volatility :
Base -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,956 -
Refractive Index @20°C :
1,477 - 1,482 -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
0,000158 mmHg @23°C -
Flash Point :
99°C (210,2°F)
-
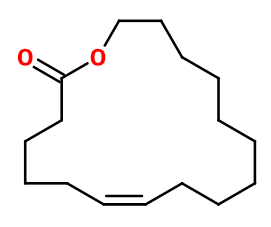
Molecular formula :
C16H28O2 -
Molecular Weight :
252,4 g/mol -
Log P :
6,51 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
187°C (368,6°F) -
Detection Threshold :
0,3 ng/l air
Chemistry & Uses
Uses in perfumery :
Ambrettolide allows to bring a sensual and carnal facets. Enhances floral-violet notes from the top.
Only used in fine fragrance because of its price. Gives a lot of power to floral notes.
Year of discovery :
Discovered in 1927.
Natural availability :
Ambrettolide is found naturally in angelica sap and in Ambrette Seeds Absolute, from which it is extracted in its natural state.
Isomerism :
Ambrettolide has a double bond, creating two possible diastereoisomers (Z) and (E). The Ambrettolide used in perfumery is a racemic mixture of these two isomers. Both diastereoisomers have a similar smell.
Synthesis precursor :
Ambrettolide is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Ambrettolide is synthesized by reaction between aleuritic acid and trimethyl orthoformate. A second step consists in reacting the intermediate product that is obtained during the previous process with acetic anhydride, and potassium hydroxide afterwards, to cyclize the second intermediate product and obtain the final product.
Stability :
Musks are very stable, as in alcoholic and in functional fragrances, exept in acid cleaners, antiperspirants and bleaches.
Other comments :
Among other musks, Ambrettolide is comparable to Helvetolide® and Velvione® thanks to its white flowers facet.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















