Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
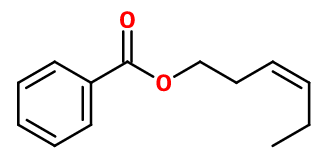
CIS 3 HEXENYL BENZOATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
25152-85-6 -
EINECS number :
246-669-4 -
FEMA number :
3688 -
FLAVIS number :
09.806
-
JECFA number :
858 -
Volatility :
Head/Heart -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
1,002 -
Refractive Index @20°C :
1.503 - 1.514 -
Optical rotation :
Data not available. -
Vapor pressure :
0.00335 mmHg @23°C 0.0015 mmHg @25°C -
Flash Point :
110°C (230°F)
-
Molecular formula :
C13H16O2 -
Molecular Weight :
204,27 g/mol -
Log P :
4,3 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
105°C (221°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Cis-3-Hexenyl Benzoate is used in floral fragrances, in white flowers accords (jasmine, ylang-ylang), floral-green, fresh notes. Also used in pear and apple notes to bring a floral facet, quite fat and juicy. Used in jasmine or gardenia floral accords, for example, to add naturalness (flower stalk effect). Also used in fruity notes. Mainly used in fine fragrance.
Year of discovery :
Data not available.
Natural availability :
Cis-3-Hexenyl Benzoate is present in Grandiflorum Jasmine Absolute in particular, from which it can be extracted in its natural state by fractional distillation. Also present in Sambac Jasmine Absolute.
Isomerism :
The use of trans-3-Hexenol for the synthesis of trans-3-Hexenyl Benzoate leads to a product with similar olfactory characteristics, but it is much less used in perfumery than cis-3-Hexenyl Benzoate. Moreover, cis-3-Hexenyl Benzoate always contains a trace of trans-3-Hexenyl Benzoate.
Synthesis precursor :
Cis-3-hexenyl benzoate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Cis-3-Hexenyl Benzoate results from the esterification reaction between benzoic acid and cis-3-Hexenol, by acid catalysis.
Stability :
Can form benzoic acid through time.
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Other comments :
Compared with Cis-3-Hexenyl Salicylate, the latter is more likely to be a reference for a solar and green note for perfumes, as Cis-3-Hexenyl Benzoate also has a leather undernote.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















