Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Anethol Naturel - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
ANETHOL | M_0050614 |
Visit website
|
Naturel | - | - |
General Presentation
-
CAS N° :
104-46-1 / 4180-23-8 -
EINECS number :
224-052-0 -
FEMA number :
2086 -
FLAVIS number :
04.010
-
JECFA number :
217 -
Volatility :
Head/Heart -
Price Range :
€€
Physico chemical properties
-
Appearance :
Colorless liquid -
Density :
0,983 -
Refractive Index @20°C :
1,557 - 1,561 -
Optical rotation :
Data not available. -
Vapor pressure :
0,0916 hPa @25°C -
Flash Point :
91°C
-
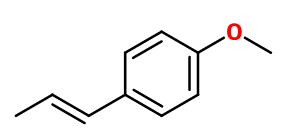
Molecular formula :
C10H12O -
Molecular Weight :
148,2 g/mol -
Log P :
Donnée indisponible. -
Fusion Point :
23°C -
Boiling Point :
236°C -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Anethole enhances fruity and floral notes by bringing a greener and more anisic facet.
Year of discovery :
Data not available.
Natural availability :
Anethole can be obtained by crystallization of Anise EO or Star Anise EO, Sweet Fennel EO or from Turpentine EO, among others. In the case of turpentine, a mixture of Anethole and beta-Caryophyllene is obtained. Then, Anethole is separated from the mixture by crystallization. Another fraction of this extraction contains both Anethole and Estragol. Therefore, a potash treatment is necessary to obtain a mixture of Anethole and alpha-Terpineol. These two molecules are separable by fractional distillation.
Isomerism :
The trans-diastereoisomer of Anethole is always the most present in its natural state. In perfumery, it is usually a mixture of the two isomers that is used. Estragole is a positional isomer of Anethole. Both molecules have an anisic note, but Estragole is more aromatic and green.
Synthesis precursor :
Anethole is a precursor to the synthesis of Anisaldehyde by oxidation.
Synthesis route :
The synthesis of Anethole is made by a Friedel-Crafts reaction using methoxybenzene and propionyl chloride. A hydrogenation followed by an acid treatment allows to obtain Anethole.
Stability :
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Other comments :
Anethole is the most common and neutral anisic note, representative of this family.
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

