Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° :
32214-91-8 -
EINECS number :
250-960-1 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless viscous liquid -
Density :
1,003 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
144°C (291,2°F)
-
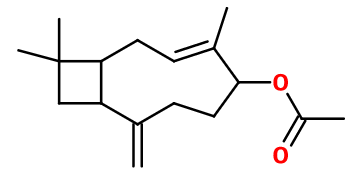
Molecular formula :
C17H26O2 -
Molecular Weight :
262,39 g/mol -
Log P :
Donnée indisponible. -
Fusion Point :
Donnée indisponible. -
Boiling Point :
152°C (305,6°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Vetyvenal® is used in vetiver reconstitutions, sandalwood notes and woody perfumes. Gives a milky and sandalwood side to an accord.
Year of discovery :
Data not available.
Natural availability :
Vetyvenal® is a compound present in trace amounts in Clove Bud EO, although it is not extracted. It is therefore the synthetic compound that is used in perfumery.
Isomerism :
Vetiveryl acetate is a constitutional isomer of Vetyvenal®. Both can be used in vetiver note reconstructions.
Synthesis precursor :
Vetyvenal® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Vetyvenal® is synthesized from an alcohol derived from Caryophyllene, reacting with acetic anhydride in an esterification reaction, in an acid medium.
Stability :
Stable in perfumes and diverse functional bases
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















