Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
VIOLIFF | VIOF-2 |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
87731-18-8 -
EINECS number :
401-620-8 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Heart -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
Donnée indisponible. -
Refractive Index @20°C :
Donnée indisponible. -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
0,02307 mmHg @23°C -
Flash Point :
110°C (230°F)
-
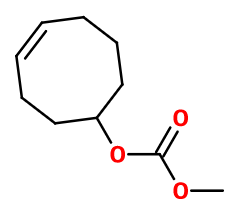
Molecular formula :
C10H16O3 -
Molecular Weight :
184,24 g/mol -
Log P :
2,9 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
-
Detection Threshold :
1,9 ng/l air
Chemistry & Uses
Uses in perfumery :
Violiff® is used in violet leaf notes, to bring a fresh, heady and aromatic effect, and for a more medicinal note.
Year of discovery :
1981
Natural availability :
Violiff® is not available in its natural state.
Isomerism :
Violiff® has two asymmetric carbons. Nevertheless, the ingredient used in perfumery is a mix of those two enantiomers.
Synthesis precursor :
Violiff® is not a precursor to the synthesis of antoher compound of olfactory interest.
Synthesis route :
Violiff® can be synthesized by an esterification reaction between methanol and a racemic mixture of carbonic [(4Z)-1-cyclooct-4-ène] acid enantiomers. This reaction has to be catalyzed by the presence of a strong acid such as concentrated sulfuric acid.
Stability :
May form acidic compounds in stability, under the effect of heat.
Terpenes are subjected to polymerization under the effect of a strong oxydation.
Other comments :
Usually, Folione® among other molecules, is used in violet leaf reproductions, because Violiff® has a less characteristic smell.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















