Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
VIOLETTYNE 10 MIP | 991805 |
Visit website
|
Molecules | - | - | |
|
|
VIOLETTYNE (MIP) | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
166432-52-6 -
EINECS number :
417-840-2 -
FEMA number :
-- -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Head -
Price Range :
Data not available.
Physico-chemical properties
-
Appearance :
Colorless to pale yellow liquid -
Density :
0,845 - 0,855 -
Refractive Index @20°C :
1.44 @20°C -
Optical rotation :
Data not available. -
Vapor pressure :
0.2809 mmHg @20°C -
Flash Point :
77°C (170,6°F)
-
Molecular formula :
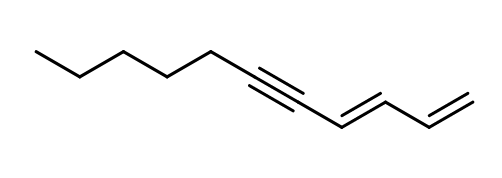
C11H16 -
Molecular Weight :
148,24 g/mol -
Log P :
5 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
-
Detection Threshold :
1,1 ng/l air
Chemistry & Uses
Uses in perfumery :
It can replace Methyl Octine or Methyl Heptine Carbonate, or be used as a new fresh green note.
A very stable and powerful green violet leaf note with a unique cucumber or bell pepper aspect.
Year of discovery :
1978 Brevet EP 0694604 registered in 1997 by Firmenich & CIE
Natural availability :
Violettyne is not available in its natural state
Isomerism :
1,3-undecadien-5-yne (Violettyne) is present as either one of its (E) or (Z) configuration isomers or as a mixture of the two. In the case of a mixture, the (E) form has to takes the majority.
Synthesis precursor :
Violettyne is a synthesis intermediary for galbanolene (1,3,5-undecatriene) and its isomers.
Synthesis route :
Full synthesis detailed here : https://doi.org/10.1002/hlca.19750580406
Stability :
Stable
Other comments :
The Violettyne is marketed while being diluted in the MIP, it is thus generally found under the name of ''Violettyne MIP ''. This dilution ensures its stability and allows a better use (very strong intensity)
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















