Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
ELEVENOL | UN-1 |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
81782-77-6 -
EINECS number :
279-815-0 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,845 -
Refractive Index @20°C :
Donnée indisponible. -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
Donnée indisponible. -
Flash Point :
83°C (181,4°F)
-
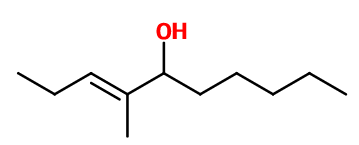
Molecular formula :
C11H22O -
Molecular Weight :
170,3 g/mol -
Log P :
3,9 -
Fusion Point :
< -50°C (< -58°F) -
Boiling Point :
228°C (442,4°F) -
Detection Threshold :
1,2 ng/l air
Chemistry & Uses
Uses in perfumery :
Undecavertol® is used to bring juiciness to fruits and a watery effect in green notes. Used in rosy, mimosa, lavender, tuberose, marine, tea, violet leaf and kiwi notes.
Year of discovery :
1981
Natural availability :
Undecavertol® is not available in its natural state.
Isomerism :
Undecavertol® has an asymmetric carbon and a double bond that gives rise to four isomers. Undecavertol® used in perfumery is a mixture of these four isomers. Aldehyde C-11 undecylic is a constitutional isomer of Undecavertol®. Its smell is however very different.
Synthesis precursor :
Undecavertol® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Undecavertol® is prepared by a Grignard reaction, using pentylmagnesium bromide (previously prepared with chloropentane and pure magnesium) and 2-methyl-2-pentenal.
Stability :
Stable in perfumes and diverse functional bases
Unstable in acid cleaners, antiperspirants and very alkaline products.
Other comments :
In comparision to other cucumber notes as (2E,6Z)-Nonadiénal, Undecavertol® has a distinctive mushroom note.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















