Photo credits: ScenTree SAS

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
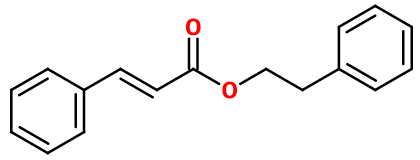
PHENYLETHYL CINNAMATE | PHCIN-1 |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
103-53-7 -
EINECS number :
203-120-3 -
FEMA number :
2863 -
FLAVIS number :
09.743
-
JECFA number :
671 -
Volatility :
Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
White crystals -
Density :
Crystal -
Refractive Index @20°C :
Donnée indisponible. -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
Donnée indisponible. -
Flash Point :
113°C (235,4°F)
-
Molecular formula :
C17H16O2 -
Molecular Weight :
252,31 g/mol -
Log P :
4,6 -
Fusion Point :
54°C (129,2°F) -
Boiling Point :
195°C (383°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Phenyl Ethyl Cinnamate is used to bring volume and as a fixative in perfumes. It enlarges the perfume and brings tenacity, especially for rosy notes.
Year of discovery :
Data not available.
Natural availability :
Phenyl Ethyl Cinnamate can be extracted from Populus balsamifera bud extract. Nevertheless, the synthetic molecule is used most of the time in perfumery.
Isomerism :
Phenyl Ethyl Cinnamate used in perfumery corresponds to the trans isomer of this molecule. The cis isomer has a relatively similar smell.
Synthesis precursor :
Phenyl Ethyl Cinnamate is not a precursor for the synthesis of another compound of olfactive interest.
Synthesis route :
Phenyl Ethyl Cinnamate is synthesized by an esterification reaction involving cinnamic acid and Phenyl Ethyl Alcohol. This reaction uses a small quantity of catalysor as concentrated sulfuric acid. A better synthesis yield can result from this reaction by using chlorocinnamic acid or cinnamic anhydride, more costly. This synthesis involves the use of the trans isomer of cinnamic acid.
Stability :
Esters may form their corresponding acid in stability
Other comments :
Phenyl Ethyl Cinnamate is almost odorless. It can only be used for its fixative effect, rather than its particular smell.
Its solubility in alcool is very week. Dipropylene Glycol is usually necessary to solubilize this molecule.
Its smell is less medicinal than the one of Ethyl Cinnamate.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















