Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
PHENYLACETIC ACID 50% DPG | ACPH50DPG-1 |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
103-82-2 -
EINECS number :
203-148-6 -
FEMA number :
2878 -
FLAVIS number :
08.038
-
JECFA number :
1007 -
Volatility :
Heart -
Price Range :
€
Physico-chemical properties
-
Appearance :
White crystals -
Density :
1,08 -
Refractive Index @20°C :
Donnée indisponible. -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
0,75 mmHg @97°C -
Flash Point :
132°C (269,6°F)
-
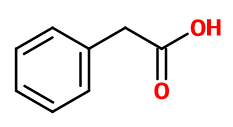
Molecular formula :
C8H8O2 -
Molecular Weight :
136,14 g/mol -
Log P :
1,4 -
Fusion Point :
77°C (170,6°F) -
Boiling Point :
265°C (509°F) -
Detection Threshold :
1 ppm (0,0001%)
Ce qui fait de lui un composé moins facilement détectable que l'Alcool phényléthylique
Chemistry & Uses
Uses in perfumery :
Phenylacetic Acid gives a honeyed and sweet touch to a rose note.
Year of discovery :
1885
Natural availability :
Phenylacetic Acid is present in Tobacco Absolute, Cocoa Absolute and chicory from which it can be extracted in small quantities. Synthetic Phenylacetic Acid remains the most produced and used in perfumery.
Isomerism :
Anisic Aldehyde and Methyl Benzoate are constitutional isomers of Phenylacetic Acid. However, their smell is not related.
Synthesis precursor :
Phenylacetic acid is the precursor for the synthesis of several esters called ''phenyl acetates '', by reacting it with an alcohol in the presence of a catalyst acid.
Synthesis route :
Phenylacetic Acid is obtained according to a synthetic route in two steps, that involves benzyl chloride with sodium cyanide, followed by a hydrolysis to obtain the corresponding acid.
Stability :
Très stable en eau de toilette.
Most of the time, the occurrence of a benzenic cycle in a molecule causes a coloration of this molecule through time
Acids may lowen a perfume’s pH. An acid pH can help preserving a perfume, but can also be the source of other raw materials modification
Other comments :
In comparison with Phenyl Ethyl Alcohol, Phenylacetic Acid brings a more butyric and honeyed facet, but less pleasant and directly reminiscent of rose.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment