Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
FLORSANTOL™ | 969344 |
Visit website
|
Molecules |


|
- | - |
|
|
OSYROL | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
41890-92-0 -
EINECS number :
255-574-7 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,9 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
>110°C (>230°F)
-
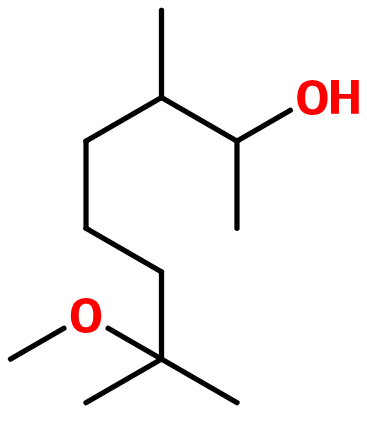
Molecular formula :
C11H24O2 -
Molecular Weight :
188,31 g/mol -
Log P :
2,76 -
Fusion Point :
8°C (46,4°F) -
Boiling Point :
230°C (446°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Osyrol is used to bring a milky and round touch to a woody note and in sandalwood accords. It is less impactant than Javanol® or Bacdanol®, and can flatten the accord if overdosed.
Year of discovery :
1972
Natural availability :
Osyrol® is not reported as found in nature, and can thus not be extracted from any plant.
Isomerism :
Osyrol® has two asymmetric carbons. This gives birth to four possible isomers for this molecule. A mixture of these isomers is used in perfumery.
Synthesis precursor :
Osyrol® is not a precursor for the synthesis of another material used in perfumery.
Synthesis route :
Osyrol® synthesis is done starting from Dihydromyrcene, reacting it with hydrochloric acid during a hydrochlorination reaction. The obtained chlorinated compound undergoes methoxylation using methyl iodide, in the presence of lithium carbonate for example. Subsequently, an epoxidation reaction is carried out, reacting the remaining alcene function with a peracid, forming the epoxide. This epoxide can be hydrogenated in the presence of Raney nickel and trimethylamine, to obtain final Osyrol®.
Stability :
Stable in perfumes and various functionnal bases.
Other comments :
Osyrol® is a milky sandalwood note that does not have a real distinctive undernote, as Javanol® and Sandalore® for example.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment