Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
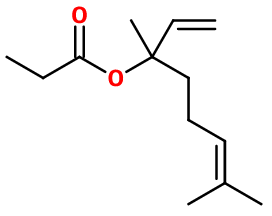
LINALYL PROPIONATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
144-39-8 -
EINECS number :
205-627-5 -
FEMA number :
2645 -
FLAVIS number :
09.130
-
JECFA number :
360 -
Volatility :
Heart -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,896 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
101°C (213,8°F)
-
Molecular formula :
C13H22O2 -
Molecular Weight :
210,32 g/mol -
Log P :
4,9 -
Fusion Point :
-20°C (-4°F) -
Boiling Point :
223°C (433,4°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Less used than Linalyl acetate, it is still used for bergamot notes, for a contribution of naturalness, a sweet note and a green facet.
Year of discovery :
Data not available.
Natural availability :
Linalyl Propionate is found in Sweet Orange EO, Lemon EO, Bergamot EO. Its laevorotatory form is present in Lavender EO and Sage EO.
Isomerism :
Linalyl Propionate used in perfumery is a racemic mixture of its two enantiomers (R) and (S). Both have a similar smell. Geranyl Propionate and Lyral® are both constitutional isomers of Linalyl Propionate. The first is more citric and rosy, and the second has a transparent floral and aldehydic note.
Synthesis precursor :
Linalyl Propionate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Linalyl Propionate is synthesized by an esterification reaction involving Linalol. This reaction may involve acetic acid, in the presence of a small amount of a concentrated strong acid, such as sulphuric acid. To improve the yield of this reaction, acetic anhydride or chloroacetic acid can be used instead of acetic acid.
Stability :
Esters may form their corresponding acid through time
Other comments :
Can contain Linalool traces.
Its smell is greener and less agrestic than the one of Linalyl acetate.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















