Photo credits: ScenTree SAS
Iso E Super®
Woody > Ambergris > Violet Flower > Cedar
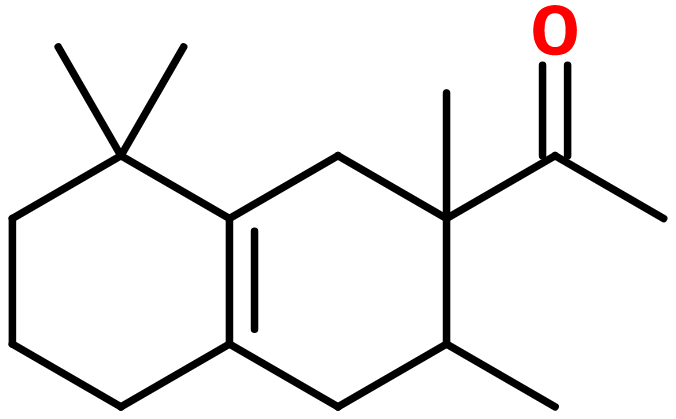
Amberonne® ; Orbitone® ; Arborone® ; 1-(2,3,8,8-tetramethyl-1,3,4,5,6,7-hexahydronaphthalen-2-yl)ethanone ; 1,2,3,4,5,6,7,8-octahydro-2,3,8,8-tetramethyl-2-acetonaphthone ; Amber fleur ; Isoamber super ; Amberfleur ; Ambergris ketone ; Amberix super ; Amberlan ; Ambroise super ; Anthamber ; Boisvelone ; Isocyclemone E ; Dimethyl myrcetone ; Hamber ; Iso gamma super ; Methyl cyclomyrcetone ; Patchouli ethanone ; Sylvamber ; Timbersilk ; Timbrone supra ; Iso velvetone

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Iso E Super - 30 Gr | - |
Visit website
|
- | - | - | |
|
|
ISO MYRCETONE | - |
Visit website
|
- | 10 grs | - | |
|
|
ISO MYRCETONE GAMMA | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
54464-57-2 -
EINECS number :
259-174-3 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,964 -
Refractive Index @20°C :
1.497 - 1.501 -
Optical rotation :
Data not available. -
Vapor pressure :
0.0015 mmHg @23°C -
Flash Point :
134°C (273,2°F)
-
Molecular formula :
C16H26O -
Molecular Weight :
234,38 g/mol -
Log P :
5,65 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
290°C (554°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Iso E Super® is used in masculine, woody and ambery notes as a woody base. Allows to bring a light violet flower note to woody accords.
Year of discovery :
Discovered in 1956. Iso E Super® was first patented in 1973, by scientists John B. Hall and James M. Sanders.
Natural availability :
Iso E Super® is not available in its natural state.
Isomerism :
Iso E Super® is composed by more than 20 isomeric molecules. The molecule called Isocyclemone in its pure state, has almost no smell, but the Iso E Super® called '' Plus '', so-called Arborone, found at between 2 and 5% in the commercialised Iso E Super®, has a very strong smell (10 000 times more powerful than Iso E Super), and is responsible for the smell of Iso E Super®. Iso E Super® Plus alone is not marketed because its production is too expensive.
Synthesis precursor :
Iso E Super® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Iso E Super® is synthesized by a Diels-Alder reaction between Myrcene and 3-methyl-3-penten-2-one in the presence of aluminum chloride. The cyclization of the intermediate product obtained is made thanks to the action of phosphoric acid. This cyclization gives birth to two isomers : Iso E Super® and Arborone, in 95/5 proportions.
Stability :
Stable in perfumes and diverse functional bases
Other comments :
The name of Iso E Super® comes from the contraction of ISOcyclomyrcetone Ethanone, and the SUPERior quality which has been made accessible since the improvement of the manufacturing process. Often called 'bois-violette'.
From 1993, each company was free to produce its own Iso E Super®. The current production of the molecule is 1800 tons per year.
IFRA
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,41 % 1,1 % 0,41 % 20 % 5,1 % 0,56 % 0,76 % 0,19 %0,0093 % Cat.5A B C DCat.6 5,1 % 0,56 % 0,76 % 0,19 %0,0093 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,67 % 0,67 %0,19 % 2,4 % 2,4 % 6,6 %0,19 % 0,19 %No Restriction Cat.10A BCat.11A BCat.12 2,4 % 6,6 %0,19 % 0,19 %No Restriction


















