Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
HELVETOLIDE® | 947650 |
Visit website
|
Molecules |


|
- | - |
|
|
Helvetolide® - 30gr | - |
Visit website
|
- | - | - |
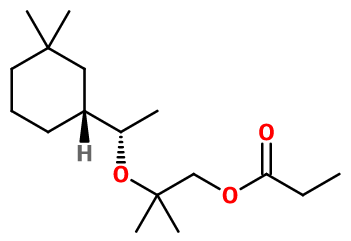
General Presentation
-
CAS N° :
141773-73-1 -
EINECS number :
415-490-5 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,94 -
Refractive Index @20°C :
Donnée indisponible. -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
Donnée indisponible. -
Flash Point :
139°C (282,2°F)
-
Molecular formula :
C17H32O3 -
Molecular Weight :
284,44 g/mol -
Log P :
5,46 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
347°C (656,6°F) -
Detection Threshold :
1,7 ng/l air
Chemistry & Uses
Uses in perfumery :
Helvetolide is used in all kinds of perfumes (but espacially in fine fragrance because of its price) to bring a heady pear note and an Ambrette Seeds Absolute base note. It can be used as a fixative, with no cheap sub-effect. It is basically used as Musk T®, but brings more volume and tenacity to the fragrance.
Year of discovery :
Discovered in 1990 by chemists Giersch and Schulte-Este. 'Helvetolide®' trademark has been published and protected by Firmenich SA since 30/04/1995 (brand N°636238). 'Helvetolide®' trademark has been published and protected by Firmenich SA since 12/12/2001 (brand N°772121)
Natural availability :
Helvetolide® does not exist on a natural state. It can't be extracted from a plant.
Isomerism :
Helvetolide® used in perfumery is a mix of two isomers, due to the presence of two asymmetric carbons inside the molecule. The first one is dextrorotatory Helvetolide® (+), less floral but more musky than the other isomer. This is why it is often preferred as the other isomers, and sometimes used alone, isolated from levorotatory Helvetolide® (-).
Synthesis precursor :
Helvetolide® can be used to synthesize Romandolide®, which is more powerful. This synthesis consists in replacing the dimethyl groupement of the ether function of Helvetolide®, by a carbonyle function, forming an ester.
Synthesis route :
Data not available.
Stability :
Stable in perfumes and in various functional bases.
Other comments :
Helvetolide® is the first acyclic musk ever used in perfumery. It participated to the opening of a new molecules category. After it, Romandolide®, Nebulone®, Edenolide® and Sylkolide® were synthesized.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















