Photo credits: ScenTree SAS
Fructone®
Fruity > Green Fruits > Butyric > Tropical Fruits
Jasmaprunate® ; Aplifine® ; Frutimety® ; Methyl Dioxolan ; Applinal® ; Ethyl 2-(2-methyl-1,3-dioxolan-2-yl) acetate ; Apple ketal ; Applinal ; Applitone ; Ethyl aceto acetate EG acetal ; Ethyl acetoacetate ethylene glycol ketal ; Ethyl-2-methyl-1,3-dioxolane-2-acetate ; Fragolan ; Frutinal ; Ketopommal ; Methyldioxolan

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Fructone® - 30gr | - |
Visit website
|
- | - | - | |
|
|
FRUCTONE | FRUC-1 |
Visit website
|
- | 10 grs | 98.0 - 100.0 |
General Presentation
-
CAS N° :
6413-10-1 -
EINECS number :
229-114-0 -
FEMA number :
4477 -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
1969 -
Volatility :
Head/Heart -
Price Range :
€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
1,085 -
Refractive Index @20°C :
1,431 – 1,435 -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
0,0477 mmHg @23°C -
Flash Point :
94°C (201,2°F)
-
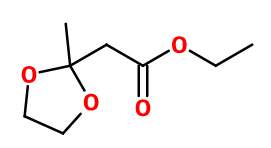
Molecular formula :
C8H14O4 -
Molecular Weight :
174,2 g/mol -
Log P :
0,98 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
95°C (203°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Fructone® is used in fruity notes for all types of fruit. Useful in rose notes for a fruity nuance, in green, aromatic and marine notes. if overdosed, addition of a fermented facet.
Year of discovery :
1938
Natural availability :
Fructone® is not available in its natural state.
Isomerism :
Fructone® does not have an isomer used in perfumery.
Synthesis precursor :
Fructone® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Fructone® and an acetal of Ethyl Acetoacetate (synthesis from formic acid and acetone in its enol form), obtained by reaction between this reagent and ethylene glycol.
Stability :
Stable in perfumes and diverse functional bases
Other comments :
Data not available.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















