Photo credits: ScenTree SAS
Evernyl®
Undergrowth > Mossy
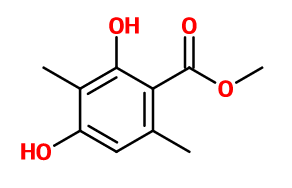
Atralone® ; Veramoss® ; Methyl 2,4-dihydroxy-3,6-dimethylbenzoate ; Ataric acid ; Atraric acid ; Crystal moss ; 2,4- dihydroxy-3,6-dimethylbenzoic acid methyl ester ; 3,6-dimethyl-beta-resorcylic acid methyl ester ; Evernilox ; Methyl 2,4-dihydroxy-3,6-dimethyl benzoate ; Methyl atrarate ; Methyl atratate ; Mousse cristal ; Musgolide ; Oakmoss #1 ; Phenomoss ; Rionyl ; Verymoss

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
Evernyl - 30 Gr | - |
Learn more
|
- | - | - | |
|
|
METHYL ATRARATE | - |
Learn more
|
- | 10 grs | - |
General Presentation
-
CAS N° :
4707-47-5 -
EINECS number :
225-193-0 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
09.623
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
White solid -
Density :
1,02 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
0.00097 mmHg @20°C -
Flash Point :
183°C (361,4°F)
-
Molecular formula :
C10H12O4 -
Molecular Weight :
196,2 g/mol -
Log P :
2,1 -
Fusion Point :
144°C (291,2°F) -
Boiling Point :
-
Detection Threshold :
0,2473 ng/l air
Chemistry & Uses
Uses in perfumery :
Evernyl® replaces oak moss in chypre and fougere compositions, for example, as it is not restricted. Used at a quite low dosage, because it is a very effective molecule. Useful to give more facets to a floral note.
Year of discovery :
1968
Natural availability :
Extraction of natural Evernyl® can be made from Oak Moss Absolute, containing Atranol and Chloratranol. These two last molecules can cause an allergenic reaction when entering in contact with the skin.
Isomerism :
Evernyl® does not have any isomer used in perfumery.
Synthesis precursor :
Evernyl® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
The synthesis of Evernyl® can be made from atraric acid in particular, which can be extracted from African plum leaf oil or Oak Moss Absolute with a good yield. The reaction is an esterification reaction with methanol.
Stability :
Very stable in perfumes and diverse functional bases
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

