Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
ETHYL OCTANOATE (ETHYL CAPRYLATE) | 441E130000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - |
|
|
ETHYL CAPRYLATE | ETCAPRY-1 |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
106-32-1 -
EINECS number :
203-385-5 -
FEMA number :
2449 -
FLAVIS number :
09.111
-
JECFA number :
33 -
Volatility :
Head -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,86 -
Refractive Index @20°C :
1,418 -
Optical rotation :
Donnée indisponible. -
Vapor pressure :
Donnée indisponible. -
Flash Point :
79°C (174,2°F)
-
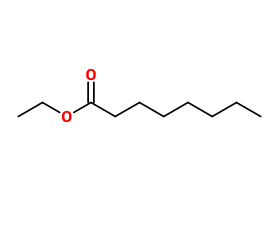
Molecular formula :
C10H20O2 -
Molecular Weight :
172,27 g/mol -
Log P :
3,84 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
208°C (406,4°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Year of discovery :
Data not available.
Natural availability :
Ethyl Octanoate is present in nature in alcohols such as Rum, Whisky, Beer, Cognac
Isomerism :
Ethyl Octanoate is a constitutional isomer of Hydroxycitronellal and Florol®. However, its smell is quite different from the latter two compounds.
Synthesis precursor :
Ethyl Octanoate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Ethyl Octanoate can be synthesized by an esterification reaction between octanoic acid and Ethanol. This reaction involves an acid catalyst such as concentrated sulphuric acid, which speeds up the reaction.
Stability :
Esters may form their corresponding acid through time
Other comments :
Data not available.
Labelling
Allergens :
This ingredient does not contain any allergen.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















