Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° :
30168-23-1 -
EINECS number :
250-078-7 -
FEMA number :
Donnée indisponible. -
FLAVIS number :
Donnée indisponible.
-
JECFA number :
Donnée indisponible. -
Volatility :
Base -
Price Range :
€€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
1,01 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
> 93°C (> 199,4°F)
-
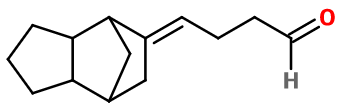
Molecular formula :
C14H20O -
Molecular Weight :
204,31 g/mol -
Log P :
4,1 -
Fusion Point :
Donnée indisponible. -
Boiling Point :
300°C (572°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
Dupical is generally used in floral notes as lily of the valley or hyacinth. It brings freshness and an interesting aquatic note. It is very strong. It has to be used in small quantities.
Year of discovery :
1969
Natural availability :
Dupical does not exist on a natural state. Thus, it can't be used as extracted from a plant.
Isomerism :
Dupical contains a few asymmetric carbons and a double bond, giving birth to many possible isomers. Nevertheless, a blend of these isomers is used in perfumery.
Synthesis precursor :
Dupical is not used for the synthesis of another molecule of olfactive interest.
Synthesis route :
Dupical can be synthesized starting from tricyclododecanone, by reacting it with a Grignard reagent called vinylmagnesium bromide, to form and alcoholic intermediate with a vinylic group. A Claisen rearrangement can then be carried out by heating the intermediary product, converting it into Dupical.
Stability :
Very unstable in stong acidic (detergents) and alkaline (liquid bleach) bases. Only stable in shampoo, candle and soap bases.
Other comments :
Synergy with Methyl Laitone, to give a lactonic effect to a floral or fruity note.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment