Photo credits: ScenTree SAS
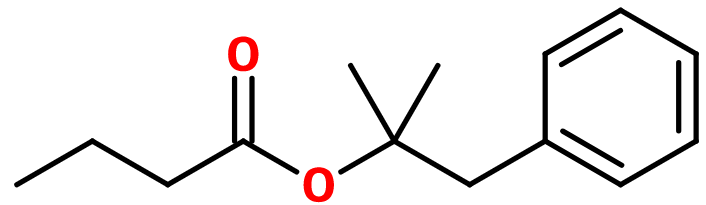
Dimethylbenzylcarbinyl butyrate
Fruity > Yellow Fruits > Rosy > Crisp Green
Dimethylbenzylcarbinyl Butyrate ; (2-methyl-1-phenylpropan-2-yl) Butyrate ; Dimethylbenzylcarbinyl butanoate ; DMBC butyrate ; DMBC butanoate ; (2-methyl-1-phenylpropan-2-yl) butanoate ; Benzylpropyl butanoate ; Dimethylbenzene ethyl butyrate

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
DMBC BUTYRATE | 85994 |
Visit website
|
Molecule | - | - | |
|
|
DMBC BUTYRATE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
10094-34-5 -
EINECS number :
233-221-8 -
FEMA number :
2394 -
FLAVIS number :
09.232
-
JECFA number :
1656 -
Volatility :
Heart -
Price Range :
€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,97 -
Refractive Index @20°C :
Data not available. -
Optical rotation :
Data not available. -
Vapor pressure :
Data not available. -
Flash Point :
94°C (201,2°F)
-
Molecular formula :
C14H20O2 -
Molecular Weight :
220,31 g/mol -
Log P :
3,99 -
Fusion Point :
< -70°C (< -94°F) -
Boiling Point :
246°C (474,8°F) -
Detection Threshold :
Donnée indisponible.
Chemistry & Uses
Uses in perfumery :
DMBC Butyrate is used in a large quantity in dry fruits and plum accords (major component of the Prunella® base). It can also be used in smaller quantity in more tasty notes as coffee, cocoa, apricot, in oriental perfumes and in floral-fruity-green rose, lily of the valley and hyacinth accords.
Year of discovery :
Data not available.
Natural availability :
Dimethyl Benzyl Carbinyl Butyrate is not available in its natural state.
Isomerism :
Dimethyl Benzyl Carbinyl Butyrate has no known isomer used in perfumery.
Synthesis precursor :
Dimethyl Benzyl Carbinyl Butyrate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Dimethyl Benzyl Carbinyl Butyrate is obtained by an esterification reaction between acetic acid and Dimethyl Benzyl Carbinol, catalysed by a small amount of a strong concentrated acid such as sulfuric acid.
Stability :
Esters may form their corresponding acid in stability
Other comments :
Hardly soluable in Propylene Glycol
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















