Photo credits: ScenTree SAS

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | MOQ | Purity |
|---|---|---|---|---|---|---|---|
|
|
METHYL SULPHIDE 'A'GRADE | 441M250000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - |
|
|
DIMETHYL SULFIDE | - |
Visit website
|
- | 10 grs | - |
General Presentation
-
CAS N° :
75-18-3 -
EINECS number :
200-846-2 -
FEMA number :
2746 -
FLAVIS number :
12.006
-
JECFA number :
452 -
Volatility :
Head -
Price Range :
€€€
Physico-chemical properties
-
Appearance :
Colorless liquid -
Density :
0,846 -
Refractive Index @20°C :
1.431 - 1.441 @25°C -
Optical rotation :
Data not available. -
Vapor pressure :
647.00 mmHg @25°C -
Flash Point :
-36°C (-32,8°F)
-
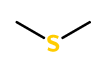
Molecular formula :
C2H6S -
Molecular Weight :
62,13 g/mol -
Log P :
0,84 -
Fusion Point :
-98°C (-144,4°F) -
Boiling Point :
38°C (100,4°F) -
Detection Threshold :
0,3 et 10 ppb
Chemistry & Uses
Uses in perfumery :
Dimethyl Sulfide is used in small quantities in exotic and red fruits, woody, green and eau fraiche notes. In low quantity, it brings a juicy and acidic effect to fruity notes, but an onion facet if overdosed.
Year of discovery :
Data not available.
Natural availability :
Dimethyl Sulfide is present in many foods consumed daily, but its synthetic version in most often used in perfumery.
Isomerism :
Dimethyl Sulfide does not have any isomer used in perfumery.
Synthesis precursor :
Dimethyl Sulfide is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
The synthesis of Dimethyl Sulfide consists of a single step: reacting two molar equivalents of bromomethane with one and a half equivalent of sodium sulfide, in a hydroalcoholic and hot medium.
Stability :
Stable in perfumes and diverse functional bases
Other comments :
Data not available.
IFRA
IFRA 51th :
This ingredient is not restricted for the 51th amendment

















